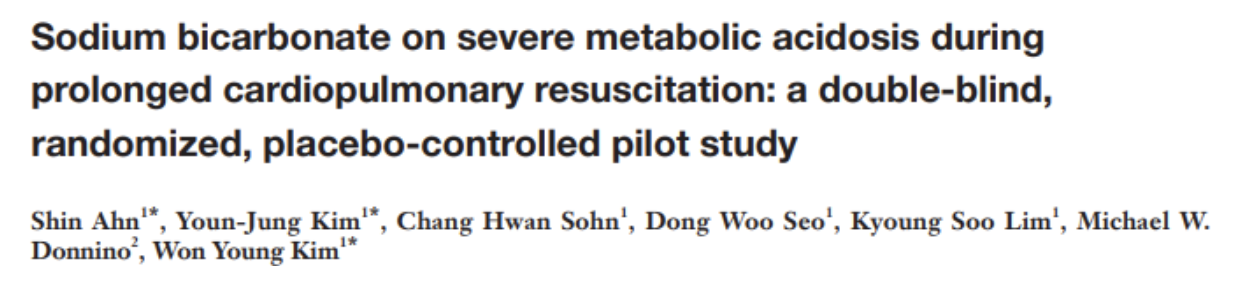
Written by: Parisa Kermani, MD (NUEM ‘23) Edited by: Alex Herndon, MD (NUEM ‘21)
Expert Commentary by: Matt Levine, MD
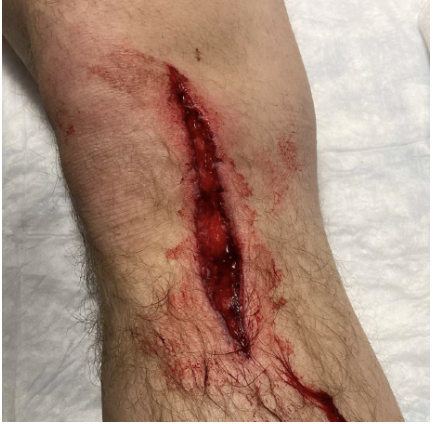
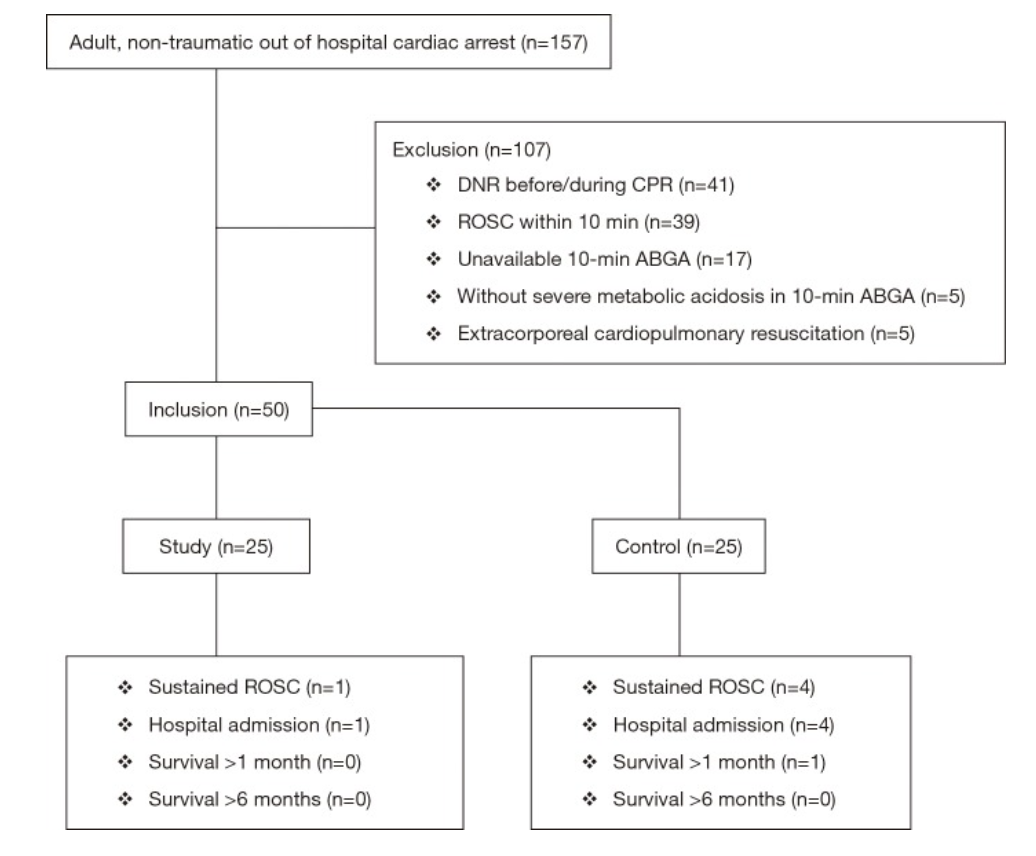
Case: A 25-year-old male comes into the ER after a saw accident at work. The patient was using a circular saw to cut wood when it slipped and the saw touched up against his knee. The patient has a 10cm linear vertical laceration over the anterior surface of his left knee (Figure 1). Bleeding is controlled. Patient ambulatory. Reporting 10/10 pain over the laceration.
What are the next best steps for evaluation and treatment of his injury?
Figure 1: Knee laceration
Background
Traumatic arthrotomy is defined as a soft tissue injury over a joint that penetrates the joint space. Violation of the joint capsule exposes the sterile intra-articular space to the environment which can result in a deep infection and sepsis. The morbidity associated with septic arthritis is high, so it is important that providers have a high index of suspicion when evaluating wounds over joint surfaces.
The knee joint is the most common joint to be affected, followed by the ankle. Penetrating injuries have a higher rate of capsule violation so a history of knives or bullets should raise suspicion, though MVCs, falls, motorcycle accidents can also result in a deep injury. The capsule has little protection lateral to the patella (Figure 2 & 3), so even if the laceration does not appear deep there is potential that it penetrates the joint space.
Figure 2: Knee capsule anatomy
Figure 3: Knee CT scan
Evaluation
Exploration: The first step of evaluation is local wound exploration. It is useful to anesthetize the wound at this point, as this will make the patient more comfortable and allow for a better exam. Irrigate the wound with sterile saline. It is extremely important to visualize the base of the wound. Using a hemostat or q-tip to probe the tissue at the base can be helpful as to not miss any tunneling segments. Keep a close eye out for bubbles, synovial fluid (appears straw colored and oily) or visible bone/tendon as all of these indicate joint involvement. It is important to note that the absence of these findings does not rule out a traumatic arthrotomy.
X-ray: Many times, next step will be to get an X-ray to look for associated fractures. Though this is not the most sensitive test for evaluating for joint space violation, if you see intra-articular air, this signifies joint involvement and no further imaging is required before calling the orthopedic surgeons. Many times, the X-ray will be normal and further testing will need to be completed. Of note, an x-ray is not required if there is no concern about injury to the bone as it is unlikely going to give a definitive answer on traumatic arthrotomy in less obvious cases.
CT Scan: As far as imaging goes, CT scan is the imaging modality of choice for traumatic arthrotomy. Though not currently the gold standard for ruling out joint violation, CT scan has become more accepted as an alternative to saline load testing the joint. Although limited, a 2013 study by Konda et al, where direct arthroscopic visualization or septic arthritis at follow-up were used as the gold standard for diagnosis, found imaging by CT scan to be 100% sensitive and specific for diagnosing traumatic knee arthrotomy. When viewing a CT scan to evaluate for traumatic arthrotomy, the presence of gas in the joint, known as pneumarthrosis, indicates intra-articular extension (Figure 4).
Figure 4: Traumatic arthrotomy on CT scan
Source: Konda et al, 2013
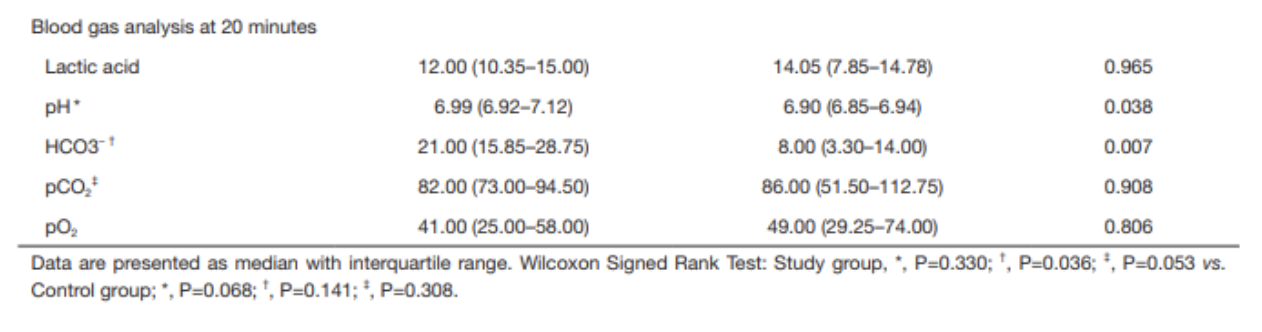
Saline Load Test (SLT): Though not strongly backed by the literature, SLT is a standard tool used to assess for traumatic arthrotomy. SLT is done by performing an arthrocentesis of the affected joint away from laceration, once confirmed in the correct space, sterile saline is injected into the joint and the laceration site is observed for extravasation. The provider should also passively range the joint while injecting to ensure greater sensitivity. Table 1 below summarizes how much sterile saline should be injected to obtain 95% sensitivity for traumatic arthrotomy. Adding methylene blue to the saline has not been proven to increase sensitivity and generally no longer recommended. The sensitivity will be highly variable based on provider experience with the procedure and patient tolerance. It is important to remember that this procedure can be exquisitely painful and special attention should be paid towards the patient’s comfort.
Table 1: Amount of saline for 95% sensitivity SLT
Because strong, conclusive literature is lacking, the choice between CT versus SLT to rule out traumatic arthrotomy will depend on many different factors including provider procedural comfort, local practice patterns, available resources and patient input.
Treatment
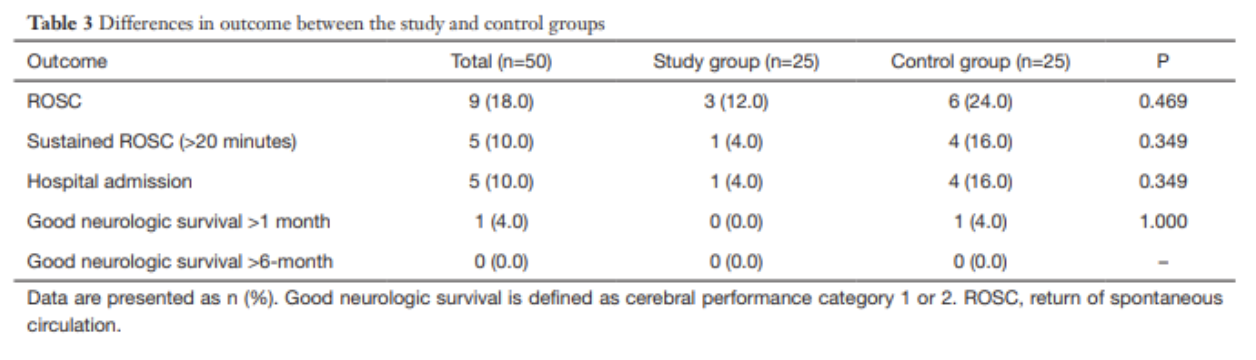
Once a diagnosis of traumatic arthrotomy is confirmed through an above modality, orthopedics should be emergently consulted. Tetanus prophylaxis should be updated and the patient should be started on an IV antibiotic that covers both strep and staph. A 1st generation cephalosporin is usually sufficient. Other antibiotics should be considered if injury is from a human/animal bite, happened underwater, or if there is concern for fecal/other contamination. Definitive treatment is joint wash out in the Operating Room.
If the above modalities do not show evidence of arthrotomy the patient’s laceration may be repaired in usual fashion. The patient should be given strict return precautions and have close follow-up for wound/joint reevaluation and suture removal.
Sources
Browning BB, Ventimiglia AV, Dixit A, Illical E, Urban WP, Jauregui JJ. Does the saline load test still have a role in the orthopaedic world? a systematic review of the literature. Acta orthopaedica et traumatologica turcica. 2016;50(6):597-600. doi:10.1016/j.aott.2016.01.004
Gittings D, Dattilo J, Fryhofer G, Martin A, Hast M, Mehta S. The saline load test is effective at diagnosing traumatic arthrotomies of the shoulder. Journal of surgical orthopaedic advances. 2019;28(4):268-271.
Gittings DJ, Fryhofer GW, Hast MW, Steinberg DR, Levin LS, Gray BL. The saline load test is effective at diagnosing traumatic arthrotomies of the wrist. Techniques in hand & upper extremity surgery. 2019;23(2):59-61. doi:10.1097
Jonathan Michael Strong. Saline Load or CT: What’s the Best Test for Traumatic Arthrotomy. Acepnow magazine. 2020; https://www.acepnow.com/article/saline-load-or-ct-whats-the-best-test-for-traumatic-arthrotomy
Konda SR, Howard D, Davidovitch RI, Egol KA. The saline load test of the knee redefined: a test to detect traumatic arthrotomies and rule out periarticular wounds not requiring surgical intervention. Journal of orthopaedic trauma. 2013;27(9):491-497. doi:10.1097/BOT.0b013e31828211f3
Konda SR, Davidovitch RI, Egol KA. Computed tomography scan to detect traumatic arthrotomies and identify periarticular wounds not requiring surgical intervention: an improvement over the saline load test. Journal of orthopaedic trauma. 2013;27(9):498-504. doi:10.1097/BOT.0b013e31828219bc
Metzger P, Carney J, Kuhn K, Booher K, Mazurek M. Sensitivity of the saline load test with and without methylene blue dye in the diagnosis of artificial traumatic knee arthrotomies. Journal of orthopaedic trauma. 2012;26(6):347-349. doi:10.1097/BOT.0b013e3182255167
Nord RM, Quach T, Walsh M, Pereira D, Tejwani NC. Detection of traumatic arthrotomy of the knee using the saline solution load test. The journal of bone and joint surgery american volume. 2009;91(1):66-70. doi:10.2106/JBJS.G.01682
Timothy D. Roberts. Traumatic arthrotomy with pneumarthrosis on plain radiograph of the knee. Western journal of emergency medicine. 2016;17(2):184-185. doi:10.5811/westjem.2015.12.29317
Expert Commentary
What a great review of traumatic arthrotomy! You now have a concise reference that teaches you everything you would probably ever need to know about this tricky diagnosis! These injuries are so uncommon that the first hurdle to overcome is actually considering the diagnosis. If you don’t consider it, then you hopefully just get lucky by a diagnostic x-ray that was ordered for other reasons!
Physical exam and exploration is indeed important but has limitations and does not rule out the diagnosis if the suspicion is high enough. The tract may be small, jagged, or there may be soft tissue destruction that limits your visualization. Be sure to inspect the wound while passively ranging the joint in question since it is often unclear the precise position of the joint (fully flexed, fully extended, or somewhere in between) when the wound occurred. This may bring the wound tract into your field of view. Ideally your exploration should be in a bloodless, painless field and documented as such.
While x-rays lack sensitivity, they are a worthwhile starting point since they are less expensive, noninvasive, readily available, and you can stop if they are positive. X-rays may also better define the extent and trajectory of the wound tract which my either heighten your suspicion or provide reassurance that the trajectory was away from the joint.
If the diagnosis is still in question, I prefer CT in most scenarios. It provides additional information about any associated fractures. CT is painless. Intra-articular air is very easy to see on CT. The downside is increased cost. Saline load testing seems to have more room for error. The joint must be properly entered. Enough fluid must be injected to fill the joint enough to cause visible extravasation. And the diagnosis can still be missed if it is forgotten to range the joint during the SLT. It is also quite painful. Consider all the patients you see who present with a painful joint effusion that has gradually accumulated. In the SLT you are giving the patient a sudden acute joint effusion. Ouch! So just be thoughtful about the route you choose to go.
Matthew Levine, MD
Associate Professor
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Kermani, P. Herndon, A. (2022, Apr 25). Traumatic Arthrotomy. [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/traumatic-arthrotomy