Brief overview of posterior reversible encephalopathy syndrome (PRES) and how it may present in the Emergency Department. This blog has been written and peer-reviewed by emergency physicians.
How to Talk Like a Neurologist
Written by: Saabir Kaskar, MD (NUEM ‘23) Edited by: Nick Wleklinski (NUEM ‘22)
Expert Commentary by: Fan Caprio, MD
Neurology Scores: LVO, NIHSS, and ICH
As first line providers, being able to effectively communicate with ancillary services and specialties is key to advancing patient care within the emergency department. When patients present with symptoms concerning for ischemic or hemorrhagic stroke, there are a variety of clinical decision tools available to help direct interventions and predict patient outcomes. Having a basic understanding of these scoring systems helps ED providers communicate more effectively with our neurology colleagues. This post highlights indications, strengths, and limitations of common stroke assessment scales used in the prehospital and hospital setting.
Cincinnati Prehospital Stroke Scale (CPSS)
The Cincinnati Prehospital Stroke Scale is a simple, easy to teach, three-part evaluation and is the most cited scale in statewide EMS protocols. Patients with one of these three findings, as a new event, will have 72% probability of ischemic stroke. If they have three of these deficits, that probability increases to 85%. Further, those scoring higher on this scale are more likely to have a large vessel occlusion (LVO) and warrant transfer to a comprehensive stroke center. One major limitation is that the CPSS does not identify features of posterior circulation strokes.
Figure 1: Cincinnati Prehospital Stroke Scale components
Predicting Large Vessel Occlusion
There are many stroke severity scales that are useful in predicting large vessel occlusion (LVO) in the pre-hospital setting. Early LVO detection is useful as these patients have better outcomes if transported to comprehensive stroke centers (CSCs) which have endovascular interventions, such as thrombectomy, readily available. Such interventions are not available at primary stroke centers (PSC). LVO screening tools include the Rapid Arterial Occlusion Evaluation Scale (RACE), the Cincinnati Prehospital Stroke Severity Scale (CP-SSS/C-STAT), the Los Angeles Motor Scale (LAMS), and the Emergent Large Vessel Occlusion Scale (ELVO). While these scales are good, none have achieved an optimal sensitivity/specificity combination which is why there is no “gold standard” test per the most recent 2019 AHA guidelines (Powers et al. Guidelines for Early Mgmt of Patients with AIS. Stroke 2019).
The Rapid Arterial Occlusion Evaluation Scale (RACE), for example, is one of these severity scales that predicts stroke caused by large vessel occlusion. It is based on the NIHSS but provides quicker assessment in the pre-hospital environment. It focuses on facial palsy, extremity motor function, head deviation, gaze deviation and aphasia or agnosia. The scale ranges from 0-9 with scores ≥ 5 being associated with detection of an LVO. RACE has a sensitivity of 85% and specificity of 68% for LVO at scores ≥ 5.
Another example of a LVO screening tool is the Cincinnati Prehospital Stroke Severity Scale (CP-SSS/CSTAT) which is important to differentiate from the CPSS outlined above. CSTAT focuses on gaze deviation, level of consciousness and arm weakness. Both RACE and CSTAT are validated in the prehospital setting and with external data sets. However, CSTAT is more convenient with fewer items to score.
EMS protocol in Chicago (Region XI), utilizes a two-tier system that first involves the Cincinnati Stroke Scale and finger to nose test. If either aspect is abnormal, then stroke severity is assessed with the 3-Item Stroke Scale (3I-SS) which assesses level of consciousness, gaze preference and motor function, scored from 0-6. If the 3I-SS score is ≥4 and the last known normal is ≤6 hours ago then the patient is transported to the closest CSC instead of the closest primary stroke center (PSC), as long as the added transport time is not >15 minutes.
National Institutes of Health Stroke Scale (NIHSS)
The NIHSS is a 11-part scoring tool and is the gold standard when assessing stroke patients in hospital (figure 3). Higher scores indicate a more severe stroke and usually correlate with infarct size on CT and MRI. Taken within the first 48 hours of acute stroke, the NIHSS helps predict three month and one-year clinical outcomes. For example, patients with a NIHSS of 1-4 have a high likelihood of functional independence and favorable outcome regardless of treatment. The NIHSS does not serve as the primary clinical guide in determining tPA administration. However, given that higher scores correlate with larger infarct size, caution is advised when considering tPA in patients with a NIHSS >22 as there is a higher risk of hemorrhagic conversion (see figure 2 for full tPA exclusion criteria). Analysis from subjects of the NINDS trials show that a NIHSS of >20 was associated with a 17% rate of intracranial hemorrhage with tPA when compared to 3% hemorrhage rate in patients with a score of <10.
Figure 2: Contraindications for tPA administration
Overall, the NIHSS is a reliable scoring tool to quicky assess the effects of stroke. Medical providers and nurses have been shown to have similar levels of accuracy when trained. Limitations include assessing posterior circulation stroke that involve gait abnormality, dizziness, or diplopia.
Figure 3: NIHSS, adopted from the American Stroke Association
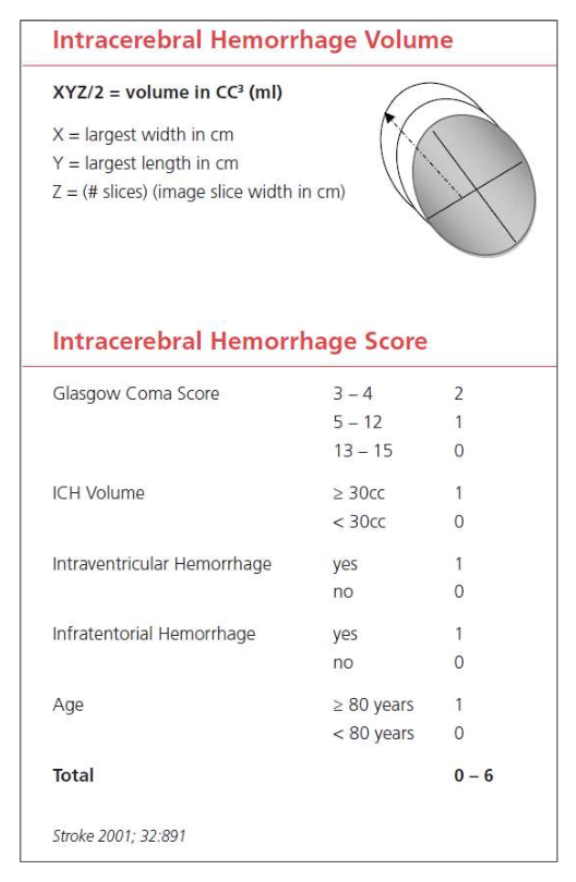
Intracerebral Hemorrhage Score (ICH Score)
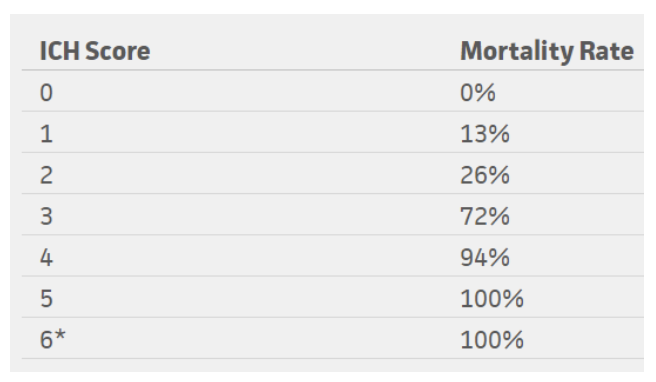
The ICH score is an important tool when evaluating a hemorrhagic stroke. This score was developed to standardize clinical grading of ICH and to improve communication between providers. This five-component scoring system (Figure 4) helps quantify ICH severity and subsequently 30-day mortality (Figure 6) with a sensitivity of 66%. It is not used to determine treatment modality. This score helps universalize the grading of ICH severity, providing a standardized language that can be used between EM providers, neurologists, and neurosurgeons. Further, this score can help providers guide goals of care conversations with patient’s families and determine appropriate level of care or transfer.
Figure 4: ICH score, adapted from the American Stroke Association
Figure 5: Mortality rates based on ICH score
*No patients in the study scored 6, but estimated 100% mortality
Conclusion
In summary, it is important to understand how to utilize these scoring tools for ischemic and hemorrhagic stroke. Knowing how to interpret pre-hospital stroke scores and how to calculate a NIHSS score accurately and quickly is helpful in not only quantifying severity but also in improving communication between providers. Improved understanding and effective use of these tools can help better advance care of our stroke patients efficiently. These tools can also remind us of the severity of the neurologic deficit we observe on clinical exam. Subsequently, this can be helpful in guiding discussions with patients and their families regarding the severity of their condition.
References
Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999; 53:126.
Goldstein, L. (2019). Use and utility of stroke scales and grading systems. Up To Date
Goldstein L, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol 1989; 46:660.
Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA Stroke Trial. Stroke 1997; 28:2119.
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001 Apr;32(4):891-7. PubMed PMID: 11283388.
Kothari RU, Pancioli A, Liu T, et al. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med 1999; 33:373.
Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke 2014; 45:87.
Schlemm L, Ebinger M, Nolte CH, Endres M. Impact of Prehospital Triage Scales to Detect Large Vessel Occlusion on Resource Utilization and Time to Treatment. Stroke 2018; 49:439.
Expert Commentary
Thanks for writing this comprehensive summary of common screening tools used in stroke patients. Having a good handle on these tools will allow you to quickly and effectively communicate with comanaging care providers. It is also important to understand how and why each scale was developed, so they can be used in the appropriate setting to expedite care in extremely time-sensitive neurologic emergencies.
Keep in mind that scales are merely screening tools and are not meant to give a definitive diagnosis. No scale is perfect, but you have highlighted some that yield the highest sensitivity and specificity for identifying a potential stroke patient. In addition to leaning on these scales as decision support tools, always use your clinical judgement. A few things to remember in addition to the neurologic symptoms:
* Strokes are potentially intervenable within the first 24 hours:
1. Up to 4.5 hours – IV-TPA / tenecteplase.
2. Up to 6 hours – Thrombectomy with LVO on vessel imaging.
3. Up to 24 hours – Thrombectomy with LVO + favorable penumbra on perfusion imaging.
* Last known normal (LKN) starts the timer to when stroke patients are eligible for intervention (not to be confused with time of symptom discovery!)
* Strokes typically cause a sudden loss of function (in contrast to positive phenomena such as convulsive movements, tingling sensation, sparkling vision, which can point away from a stroke diagnosis)
* In patients with prior deficits, ask which symptoms are new or different in comparison to their baseline.
The NIHSS is widely accepted as THE stroke severity scale, and it has many strengths and some pitfalls. The NIHSS was initially developed to be used in research, and, as mentioned here, was designed to be reproducible between various groups – physicians, nurses, research staff. Higher scores correlate with bigger infarct volume. The NIHSS is not an accurate scale in that it does not necessarily capture each patient’s deficits, omitting brain functions such as gait, distal limb dexterity, and cognition. It also scores higher for dominant (L) hemispheric functions as many points depend on language function.
When screening for large vessel occlusion, remember key brain structures and functions from the L MCA, R MCA, and posterior circulation. Looking for cortical signs can be very helpful to identify larger stroke syndromes: aphasia, neglect, gaze deviation, visual field deficit.
Last but not least, keep in mind that hemorrhagic strokes (intracerebral hemorrhage, subarachnoid hemorrhage) account for about 15% of all strokes. The same screening tools for acute neurologic symptoms can be used to identify these patients, though they more often have concurrent headache or LOC than ischemic strokes (due to increased ICP and irritation from blood products). For SAH, two scales are commonly used to describe the clinical and radiographic severities: Hunt-Hess (surgical risk index) and modified Fisher scales (risk index for developing vasospasm).
Figure 1: Hunt-Hess Scale
Figure 2: Modified Fisher Scale
Fan Caprio, MD
Assistant Professor of Neurology (Stroke)
Department of Neurology
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Kaskar, S. Wleklinski, N. (2021, Oct 25). How to Talk Like a Neurologist. [NUEM Blog. Expert Commentary by Caprio, F]. Retrieved from http://www.nuemblog.com/blog/neuro-scores
Other Posts You May Enjoy
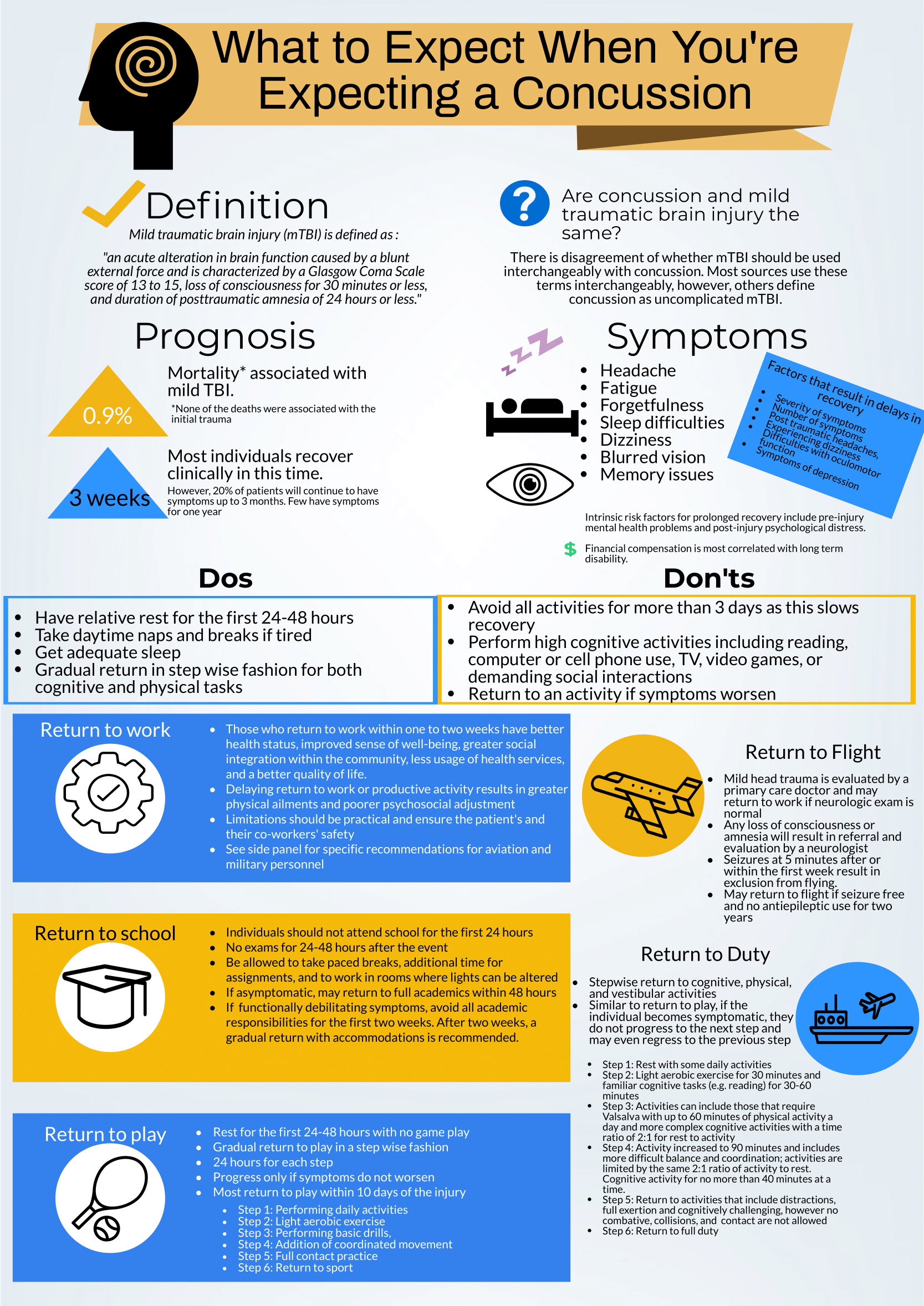
What to expect when you're expecting a concussion
Written by: Kelsey Green, MD (NUEM ‘23) Edited by: Jordan Maivelett, MD (NUEM ‘20) Expert Commentary by: Jake Stelter, MD
Expert Commentary
This is a great review of anticipatory guidance when counseling patients who have been diagnosed with a concussion. As noted, “mild traumatic brain injury (mTBI)” is often used synonymously with “concussion.” A better way to conceptualize this is to view concussion as a form of mTBI, realizing that mTBI can represent a spectrum of conditions. One of the most important treatments of concussion from the Emergency Department (ED) perspective is to counsel patients on what to expect and how to best control their symptoms. Concussions can present with a wide range of symptoms as detailed and can be quite distressing and disruptive to patients. As correctly pointed out, the presence of vestibular symptoms (i.e. dizziness or gait instability) as well as pre-existing mental health diagnoses, such as depression or anxiety, are associated with a protracted symptom course. Setting expectations of the symptoms they may develop and the possible timeline of symptom duration is important for patients as they manage their condition. Early conservative treatment with adequate sleep and relative cognitive and physical rest will help manage and reduce the intensity of symptoms. In our current society, it is nearly impossible to completely avoid screens and reading. Hence, “everything in moderation” is appropriate when counseling these patients. If the patient has to work at a computer, advise them to take frequent breaks for at least 10 minutes for every 30 minutes of screen time. In addition, it is recommended that patients with a concussion avoid alcohol. It is also advisable to avoid excessive caffeine. However, if a patient already uses caffeine on a daily basis, they should not stop completely, as that can lead to withdrawal headaches. Over-the-counter pain relievers, such as naproxen, ibuprofen or acetaminophen are appropriate for headache treatment, provided there are no contraindications to use.
There are multiple return-to-learn, -work and -play protocols that have been published. This is particularly applicable to athletes who have sustained a sport-related concussion (SRC). Most schools and athletic programs have protocols that have been developed in conjunction with athletic trainers and team physicians. It is important to remember that as an ED provider, you should not clear a patient to return to play. That process needs to be conducted by the school athletic trainer in collaboration with the team physician after they have had the opportunity to evaluate the patient. You should consider referring your concussion patients to a Primary Care Sports Medicine or Neurology provider for follow-up if they do not have a team physician to visit.
There are multiple free resources available to providers who are interested in learning more about concussion and educating patients. The Sport Concussion Assessment Tool – 5th Edition (SCAT5) is an in-depth evaluation tool that is often used by Sports Medicine clinicians when evaluating the extent and severity of a patient’s concussion syndrome. These resources are listed here:
References
American Medical Society for Sports Medicine position statement on concussion in sport:
https://bjsm.bmj.com/content/53/4/213
SCAT5:
https://bjsm.bmj.com/content/bjsports/early/2017/04/26/bjsports-2017-097506SCAT5.full.pdf
Jacob Stelter, MD
Emergency Medicine, Primary Care Sports Medicine
Division of Emergency Medicine
NorthShore University HealthSystem
How To Cite This Post:
[Peer-Reviewed, Web Publication] Green, K. Maivelett, J. (2021, Feb 14). What to expect when you're expecting a concussion. [NUEM Blog. Expert Commentary by Stelter, J]. Retrieved from http://www.nuemblog.com/blog/concussion.
Non Contrast CT Head for the EM Physician
Written by: Philip Jackson, MD (NUEM ‘20) Edited by: Logan Weygandt, MD, MPH NUEM ‘17) Expert Commentary by: Katie Colton, MD
Relying on in-house radiology reads of imaging is a habit that EM trainees are encouraged to avoid, but one that can be appealing when practicing in a busy, large academic facility with 24-hour radiologist staffing. By reading one’s own images, not only do EM physicians gain skills in diagnostic radiology, which they can employ when an attending radiology read is not readily available but more importantly, the EM physician can correlate history and physical with imaging and help detect subtle pathology. Recent studies have shown that even attending EM physicians are often deficient in reading non-contrast CT scans of the head, however, with minimal training residents have been shown to make significant improvements. [2,3]
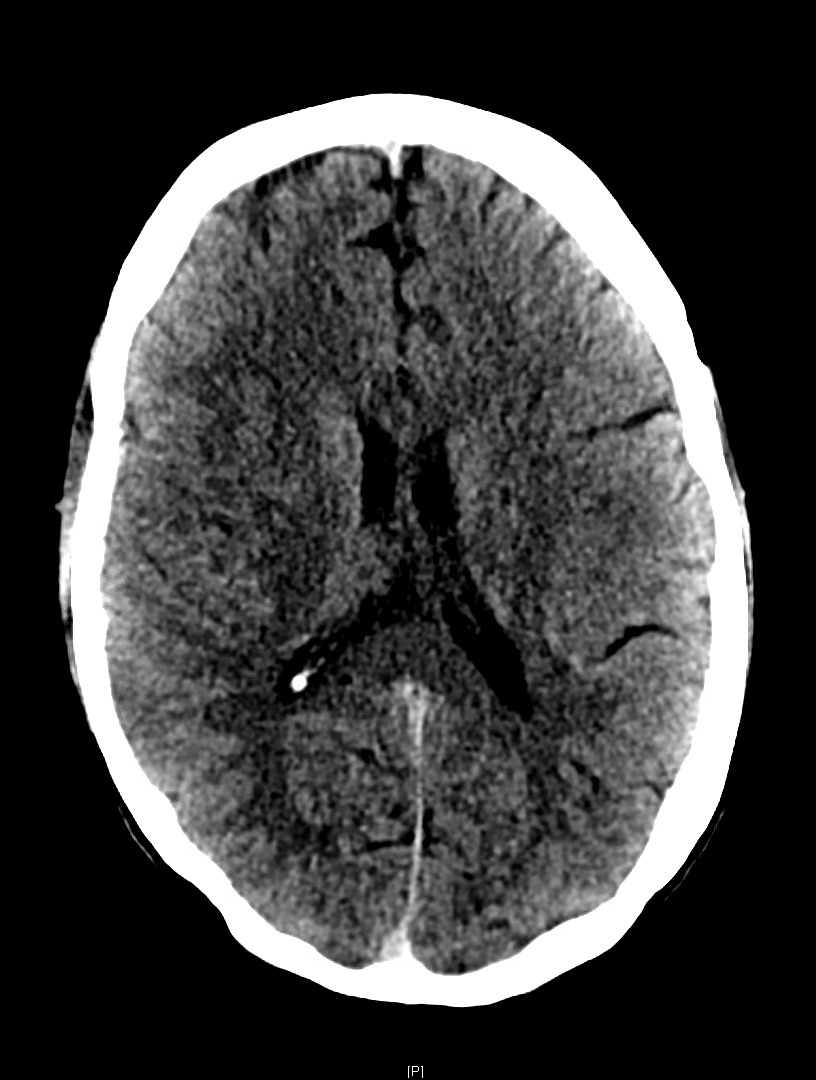
An elderly male with a history of hypertension and Fuch’s corneal dystrophy presented to our ED the morning after developing acute on chronic worsening of the blurry vision in his R eye. He suffered from persistent blurry vision but stated that it had suddenly worsened while watching TV the previous night. He then developed a left-sided occipital headache that continued through the following morning. He also noticed that his thinking was “cloudy” and despite being a healthcare professional could not describe his own medical history or list of medications. He described blurriness especially on the right. On visual field confrontation, the patient was found to have a binocular R sided superior quadrantanopsia. The rest of his neurologic exam was unremarkable. As these findings were concerning for stroke specifically in the left temporooccipital region known as Myer’s loop, we obtained a STAT non-contrast head CT.
As the so-called green arrow-signs on the CT image indicate, there was indeed a significant amount of cerebral edema present in the L temporal lobe white matter, which contains the anterior optic radiations carrying information from the R superior visual field and corresponds to our patient’s deficit. Upon discovering this lesion, our team immediately called our radiology colleagues who confirmed our concern for an acute ischemic infarct.
Like any other task in the ED, reading a head CT should be conducted as efficiently and accurately as possible using a standardized approach. EM residents have been found to be somewhat deficient in our ability to evaluate noncontrast head CTs; however, studies have shown that with adequate training, our skills can significantly improve. [3] Perron et al describe the simple but systematic approach “Blood Can Be Very Bad.” This mnemonic reminds residents to examine for the presence Blood, the shape and consistency of the Cisterns, the texture of the Brain parenchyma, the Ventricles, and the presence of fractures and symmetry of the Bony structures.
Blood: In a non-contrast CT, blood will appear as hyperdense (bright/white) fluid. As blood ages over weeks, it will become increasingly hypodense (darker). Blood will present in one of the four following ways:
Subarachnoid hemorrhage - A dreaded complication of trauma, a ruptured aneurysm, or an arteriovenous malformation can lead to blood pooling in gravity-dependent areas correlating with the particular arterial defect. Rupture of the anterior communicating artery (ACA) will distribute blood in and around the interhemispheric fissure, suprasellar cistern, and brainstem. Rupture of the middle cerebral artery (MCA) will distribute blood in the Sylvian and suprasellar cistern, while the posterior cerebral artery (PCA) will also distribute in the suprasellar cistern.
Subdural hemorrhage (SDH) – Caused by rupture of the bridging veins, SDHs will present as a crescentic lesions that often cross suture lines. SDHs can be acute, chronic, or mixed, and thus will have varying degrees of density.
Epidural Hemorrhage - Another serious complication of trauma, epidural hemorrhages will present as a lenticular (biconvex) areas of hyper-attenuation. Caused by arterial laceration, with the most common being the middle meningeal artery, epidural hemorrhages can rapidly expand and cause significant and rapid mass effect. Early identification is thus crucial to reducing mortality from these injuries.
Intraparenchymal/intraventricular hemorrhage - Often the result of hypertensive disease in elderly patients or as hemorrhagic strokes, intraparenchymal hemorrhage will most often be located in the basal ganglia. Amyloid angiopathy (associated with Alzheimer’s dementia) often presents as wedge-shaped areas of hemorrhage in the outer cortex. Trauma leading to brain contusion can also present with intraparenchymal hemorrhage. All intraparenchymal hemorrhages (as well as subarachnoid hemorrhages) can potentially rupture into ventricles causing intraventricular hemorrhage and resultant hydrocephalus.
Cisterns: Cisterns are spaces surrounding and cushioning brain matter with cerebrospinal fluid. Each of the four major cisterns should be examined for blood or signs of mass effect: the sylvan fissure (in between temporal and parietal lobes), the circummesencephalic or peripontine cistern, the suprasellar (surrounding the circle of Willis), and the quadrigeminal (atop the midbrain).
Brain matter: Always examine the gyri for and for distinct grey-white matter differentiation. Ischemic strokes, as in our case, will present with blurring of the grey-white differentiation and cerebral edema (areas of hypodensity). Early strokes may not be apparent on CT, but after 6 or more hours hypodense lesions should be present with maximal edema occurring approximately 3-5 days after the event. Always examine the falx for midline shift through multiple slices.
Ventricles: Examining the third and fourth ventricles is crucial in determining the presence of blood hydrocephalus (dilation) or mass effect (asymmetry).
Bone: The bony structures of the head should all be examined for fractures, especially depressed skull fractures, which usually denote intracranial pathology. Also, examining the sphenoid, maxillary, ethmoid, and frontal sinuses for air fluid levels should raise suspicion for a skull fracture. Separate bony windows are available for close examination of these high-density structures. [1]
As our case illustrates, it is crucially important for EM physicians to interpret non-contrast CT scans in a systematic and accurate manner. Clinical correlation is a distinct advantage that we, as emergency physicians, possess and it should be exploited to allow for timely and effective patient care.
Expert Commentary
Thanks to Drs. Jackson and Weygandt for this great primer to the emergent head CT. One of the obvious challenges of EM is the breadth of pathology we see, and so having a strategic approach like this one will reveal most of the emergent diagnoses we are looking for. I will never be a radiologist, but nothing is faster than looking at my own scan. A few thoughts: I start by scrolling a scan through quickly to identify obvious pathology (a bleed, midline shift, etc.) and then try to actively redirect my attention back to a systematic approach. It is easy to hone in on the obvious abnormality and miss smaller but crucial clues. Go through the same progression every time. Get comfortable with finding different windows for your imaging. If you only look in a brain window, you’ll miss critical diagnoses. Symmetry is your best friend - until it is not. We are remarkably good at picking out asymmetry when looking at imaging, which reveals many of the emergent diagnoses, but keep some of the symmetric processes in the back of your mind. Many of these can wait for a radiologist’s fine- tooth comb, but a few stand out. Get used to finding the basilar artery, particularly in your unconscious patient; an acute occlusion in this midline structure is potentially devastating but quick intervention is life-saving. Similarly, acute hydrocephalus merits immediate intervention that can lead to dramatic clinical improvement. Bilateral or midline subdural hemorrhage can also be easily missed; finding these requires a level of comfort with windowing the images and identifying abnormal CSF spaces.
Katie Colton, MD
Instructor, Feinberg School of Medicine
Department of Neuro Critical Care and Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Philip, J. Weygandt, L. (2020, Feb 10). Non Contrast CT Head for the EM Physician. [NUEM Blog. Expert Commentary by Colton, K]. Retrieved from http://www.nuemblog.com/blog/non-contrast-ct-head-for-the-em-physician
Other Posts You May Enjoy
References
Adams, James, and Erik D. Barton. Emergency Medicine: Clinical Essentials. 2nd ed. N.p.: Elsevier Health Sciences, 2013;633-644.
Jamal K, Mandel L, Jamal L, Gilani S. 'Out of hours' adult CT head interpretation by senior emergency department staff following an intensive teaching session: a prospective blinded pilot study of 405 patients. Emergency medicine journal : EMJ. 2014;31(6):467-470.
Perron AD, Huff JS, Ullrich CG, Heafner MD, Kline JA. A multicenter study to improve emergency medicine residents' recognition of intracranial emergencies on computed tomography. Annals of emergency medicine. 1998;32(5):554-562.
Mayfield Brain & Spine. "Visual field test." Visual Field Test | Mayfield Brain & Spine. N.p., n.d. Web. 19 Dec. 2016.
Emergency Guide to Stroke Neuroimaging
Written by: Justin Seltzer, MD (PGY-3) Edited by: Luke Neill, MD (PGY-4) Expert commentary by: Babak Jahromi, MD, PhD
According to the CDC, an ischemic stroke occurs approximately every 40 seconds in the US, with nearly 800,000 documented cases annually.[1] This, combined with an effective national stroke symptom public education program, has resulted in a large number of patients presenting to emergency departments for evaluation of stroke or stroke-like symptoms. Essential to this initial evaluation is neuroimaging, which in the emergency department is mainly CT based.
However, despite frequent use, many emergency physicians are not familiar enough with stroke imaging to interpret images on their own. A prior post addressed the basics of reading a complete head CT, which you can find here. The goal of this article is to discuss the indications and limitations as well as to provide a basic guide to interpretation of noncontrast CT imaging of the brain (NCCT), CT angiography (CTA) of the head and neck, and CT perfusion (CTP) imaging in acute stroke evaluation.
Acute stroke imaging is obtained in the emergency department for two purposes.
To evaluate rapidly for thrombolysis contraindications like hemorrhage and certain pathology such as vascular malformations and aneurysms. Thrombolysis has a high therapeutic benefit in stroke patients, with a number needed to treat of 10 within 3 hours of symptom onset and less than 20 if administered within 4.5 hours.[2,3] In addition, door to needle time of less than one hour is an established benchmark and quality measure.[3]
To identify a causative vascular lesion, which may or may not be amenable or contraindicatory to thrombolysis
Non-Contrast Head CT
NCCT is usually the first imaging modality obtained in the acute evaluation for stroke. Within the thrombolysis window (<4.5 hours), however, this scan is far more likely to detect hemorrhage than infarction. Chalela, et al., reviewed 356 patients evaluated for stroke symptoms at a single center over 18 months. They showed a sensitivity of 89% for detection of acute intracranial hemorrhage; conversely, the sensitivity for ischemic strokes less than 3 hours old was 12%, 16% for those older than 12 hours, and an overall sensitivity of 16%.[4] These findings are consistent with other studies and highlights the limitations of NCCT in acute stroke imaging.
Despite the poor sensitivity for acute infarction, there are a few ways to improve detection. Windowing adjustments can enhance grey-white matter differentiation, as loss of this in an area anatomically associated with the presenting deficit is suggestive of acute infarction. A window width and center of approximately 50 each achieves adequate grey-white differentiation (Figure 1). Additionally, asymmetric, hyperdense section of cerebral vasculature, known as the “dense vessel” sign, is also highly suggestive of middle cerebral artery (MCA) occlusion.[5] As a side note, IV contrast should not be used outside of angiography to “enhance” the image as it may extravasate into the ischemic parenchyma mimicking hemorrhage.[6]
Figure 1. NCCT of the brain in an acute right M1 occlusion with a last known well time was approximately 13 hours before. Windowing set at C50/W50 for improved grey-white differentiation. Official read: “A diffuse asymmetric hypodensity and subtle loss of gray-white matter differentiation in the right frontal and parietal region is highly concerning for an acute right MCA stroke.”
CT Angiography of the Head and Neck
The role of CTA in acute stroke evaluation is to identify the culprit vascular lesion and is an excellent addition to the emergent evaluation of acute ischemic stroke. A 2014 pooled analysis of 21 studies from 1993 to 2013 showed CTA has a sensitivity of 83.2% and specificity of 95% with a 97.1% negative predictive value for greater than 50% cerebral vascular stenosis;[7] a 2017 pooled analysis of 7 studies from 2003 to 2012 broadly reported a sensitivity of 93% and specificity of 100% for acute ischemic stroke.[8] CTA of the neck is also obtained to evaluate the contributing cervical vasculature. Since interpretation of angiography is dependent on knowledge of the relevant anatomy, the key structures are reviewed below. If a more detailed review is desired or necessary, several neuroanatomy texts may be found in the references.
The major cerebral vasculature is supplied by the bilateral internal carotid arteries (ICA; “anterior circulation”) and the paired vertebral arteries (VA) that merge to form the basilar artery (BA; “posterior circulation”). The anterior circulation dominates perfusion of the cerebral hemispheres apart from the occipital lobe. The posterior circulation feeds the remaining structures, mainly the occipital lobe, cerebellum, and brain stem.
Figure 2. CTA of the neck showing bilateral patent CCAs and VAs.
Anterior circulation
The anterior circulation starts with the ICA, which branches from the common carotid artery (CCA) in the upper neck at around the level of the fourth cervical vertebra. (Figures 2, 3). The ICA has four parts with seven defined segments; in general, segments assist with lesion localization and are provided in parenthesis. The cervical part (cervical segment, C1) is first and enters the skull at the carotid foramen (Figure 5). It is distinguished from its companion external carotid artery by a lack of extracranial branching. Once in the skull, the petrous part (petrous segment, C2) traverses the carotid canal within the petrous portion of the temporal bone (Figure 5). Moving out of the temporal bone, the ICA then crosses into the cavernous sinus, where it is known as the cavernous part (lacerum segment, C3, cavernous segment, C4, clinoid segment, C5). Navigating the bony turns in this area results in a characteristic curvature known as the “carotid siphon” (Figure 6). From here, the vessel passes through the dura, where it becomes the cerebral or supraclinoid part (ophthalmic segment, C6, communicating segment, C7) and gives off the ophthalmic, posterior communicating, and anterior choroidal arteries; these posterior communicating arteries (PCommA) run to the ipsilateral posterior cerebral arteries (PCA), thus connecting the anterior and posterior circulations and forming part of the circle of Willis (Figure 7). At the terminus, the internal carotid arteries bifurcate into the bilateral anterior cerebral arteries (ACA) and MCAs. Acute ICA lesions can cause dramatic symptoms due to restricted blood flow to the ipsilateral ACA and MCA and are large vessel occlusions.[9-12]
Figure 3. CTA of the neck showing the bilateral carotid bifurcations. Artifact from metal in the patient’s teeth.
Figure 4. CTA of the neck showing patent bilateral ICAs as well the the bilateral VAs entering the foramen magnum
The ACAs run between the frontal hemispheres in the longitudinal fissure and supply a large portion of the medial cerebral structures such as the medial frontal and parietal lobes as well as the basal ganglia and parts of the internal capsule. They are smaller than the MCAs and their course is recurrent frontal-occipital and inferior-superior, which can make visualization in the axial plane difficult to appreciate. The paired arteries are connected by the anterior communicating artery (ACommA) early in their course which is the final connection completing the circle of Willis (Figure 7). Lesions within the A1 segment, which runs from the carotid terminus to the ACommA are considered large vessel occlusions though may be better tolerated due to collateral flow through the anterior communicating artery.[9,10,12]
Figure 5. CTA of the head showing the ICAs as they enter the skull and traverse the petrous portion of the temporal bone.
Figure 6. CTA of the head showing the ICA as it traverses the cavernous sinus; the carotid siphon is well visualized on the left.
The MCAs provide circulation to the remaining frontal and parietal lobes, basal ganglia, and internal capsules, as well as portions of the temporal lobes. They are larger and therefore more easily visualized than the ACAs (Figure 7). A lesion of the M1 segment, which runs from the carotid terminus to the bifurcation into the M2 segments, is considered a large vessel occlusion (Figures 8, 9).[9,10,12]
Figure 7. CTA of the head showing an intact circle of Willis
Figure 8. CTA of the head showing an acute right M1 occlusion in the axial plane
Figure 9. Coronal MIPS of the same vascular occlusion noted in Figure 8 with clear deficit on the right compared with the left.
Posterior circulation
The posterior circulation starts with the VAs, which are subclavian branches that traverse the cervical spine via transverse foramina (Figures 2, 3). Prior to joining, each vertebral artery gives off an ipsilateral posterior inferior cerebellar arteries (PICA) as well as the contributing vessels that form the anterior and posterior spinal arteries. Upon entering the skull via the foramen magnum, the bilateral vertebral arteries join to form the basilar artery at about the level of the medullo-pontine junction (Figures 4, 5, 6). As the basilar artery moves superiorly it gives off the bilateral anterior inferior cerebellar arteries (AICA), multiple bilateral small perforating pontine arteries, the bilateral superior cerebellar arteries, and then finally terminates with a bifurcation into the bilateral posterior cerebral arteries (PCA). As noted prior, these PCAs connect with the ipsilateral posterior communicating arteries from the anterior circulation (Figure 7). Vertebral, basilar, and early posterior cerebral artery occlusions are considered large vessel occlusions but there is, as of now, limited data on mechanical thrombectomy in these territories.[9,10,12,13]
Application
Reading the scan itself is fairly straightforward based on the vascular anatomy. We recommend starting caudally (usually the aortic arch) in the axial plane and tracing all four cervical vessels cranially until they form the circle of Willis and from there extend out into the major branches. The coronal plane is particularly useful for evaluation of the anterior cervical vessels and the MCAs. Significant asymmetry or loss of contrast opacification in vascular beds anatomically consistent with the presenting symptoms should be considered strokes until proven otherwise. Make note of vascular abnormalities such as significant carotid stenosis, aneurysms, and malformations.
Additional 2-D and 3-D post-processing images may also be provided. The most common is maximum intensity projection (MIP), which highlights high density structures over low density; this allows for improved visualization of the contrast enhanced vasculature at the expense of the surrounding brain tissue. However, MIP images can be falsely negative and should not be used alone for primary vascular evaluation.[14]
CT Perfusion
Though less common than CTA, CTP may also be acquired in the emergency setting to evaluate for territorial changes in cerebral blood flow suggestive of stroke. It is particularly valuable for identifying core infarct and salvageable ischemic penumbra and is becoming an important part of interventional decision making. It has a similar sensitivity and specificity for acute ischemic stroke as CTA, its use has been validated in multiple interventional stroke studies, and it has been shown to predict core infarct size accurately compared to the gold standard MRI.[7,8,15]
Basic concepts
While the specifics of CTP are complex and beyond the scope of this article, there are a few important concepts. CTP operates under the “central volume principle,” which is represented by the equation CBF = CBV/MTT and defines the relationship between cerebral blood volume (CBV; volume of flowing blood in a set volume of brain tissue), blood flow (CBF; per time unit rate of flowing blood in a set volume of brain tissue), and mean transit time (MTT; average time for blood to transit a set volume of brain tissue). To illustrate this concept, imagine an acute arterial occlusion. The obstruction causes an immediate increase in MTT due to slowed arterial flow through the affected tissue. To maintain CBF a local compensatory vasodilation occurs, increasing CBV. However, this vasodilation may not be able to compensate for rising MTT, causing a progressively inadequate CBF that may result in infarction.[5,16]
Algorithms translate detected changes in MTT, CBV, and CBF into images that can be used in clinical decision-making. MTT is obtained by measuring the movement of contrast through the affected tissue; this also gives a value known as Tmax, which is the time to achieve peak contrast density. CBV and CBF are calculated relative values (rCBF, rCBV) and based off of the surrounding normal tissue. Composite metrics, such as mismatch ratio, the ratio of penumbra to the core infarct volumes, and mismatch volume, the penumbra volume minus the core infarct volume, are also generated.[11] Though there is no set rule, there is evidence that thrombolysis benefit is maximized and hemorrhage risk minimized with a mismatch ratio of 1.8 or greater, a mismatch volume of 15ml or greater, and a core infarct volume less than 70ml.[17]
Figure 10. Illustrative CTP report for the same acute right M1 occlusion from Figures 8 and 9 showing the core infarct (purple) and associated penumbra (green). Note the large mismatch volume and ratio, indicating a relatively small core infarct relative to the threatened penumbra.
Application
These values are then made into “parametric maps” superimposed onto axial CT slices, allowing for visual identification (Figures 10, 11). Different software may present the values and parametric maps differently; note that our institution uses RAPID (iSchemaView, Menlo Park, CA) and our example figures were generated by this software. Using Figure 10 as an example, we see purple and green areas as well as different volumes and ratios. The purple area corresponds to the volume of tissue with a rCBF less than 30% of the unaffected, healthy tissue and is considered the core infarct area. The green area corresponds to the volume of tissue with a Tmax longer than six seconds and is considered the ischemic penumbra. Though these threshold values were used and validated by the SWIFT PRIME and EXTEND-IA trials, they are not definitive or universal.[15,18] Familiarization with an institution’s software and threshold values is vital to interpreting CTP properly.
Importantly, CTP can be abnormal in other situations such as with chronic infarcts, vasospasm from subarachnoid hemorrhage, microvascular ischemia, and cerebral changes associated with seizure and feeding vessel stenosis.[16] Always interpret CTP in the context of the other imaging findings and anatomic consistency.
Figure 11. Illustrative CTP report for the same acute right M1 occlusion from Figured 8, 9, and 10 showing territorially increased MTT with subtle reduction in CBF and a small area of asymmetrically elevated CBV in the area corresponding to infarction in Figure 10. This figure visually highlights the relationships between rCBV, cCBF, MTT, and Tmax.
Take Away Points
CT is the primary source of neuroimaging in the emergency department evaluation of stroke patients. NCCT is poor at detecting early acute infarcts directly, however it is excellent for hemorrhage detection. Use of CTA can demonstrate causative vascular lesions and addition of CTP can further delineate ischemia and determine how amenable it might be to intervention. Not all lesions identified by CTA and CTP will be amenable to thrombolysis or thrombectomy, but these are usually the only time effective ways available to emergency physicians to identify those that might be. Educating emergency physicians about these imaging modalities can both improve patient care through more rapid diagnosis in suspected stroke cases as well as help to streamline communication and treatment planning with consulting neurologists and neurointerventionalists.
Expert Commentary
This is a well-written synopsis of modern neuroimaging used today’s ED for workup and emergent treatment of acute stroke. The reader should keep in mind that the primary thrust of this blog segment is on acute ischemic stroke - while advanced CT imaging (i.e. CTA) also has a crucial role in hemorrhagic stroke, this is more thoroughly addressed elsewhere.
Practically speaking, today’s CT/CTP/CTA is to suspected stroke what an EKG is to chest pain in the ED. While confirmatory tests (MRI for stroke, troponin for MI) take more time, all actionable data depends on the initial CT/CTP/CTA in acute stroke. I would also categorize the purpose of acute stroke imaging in the ED into two categories, but with perhaps broader brush-strokes:
Determine if stroke is ischemic or hemorrhagic (“blood or no blood on CT”), and
Determine the next course of action:
If ischemic, do temporal and anatomic criteria mandate IV tPA, endovascular thrombectomy, both, or neither,
If hemorrhagic, is there mass effect and/or an underlying vascular lesion (arterial or venous) that mandates urgent intervention beyond best medical care.
While NCCT is sufficient to determine whether to proceed with IV tPA in the 0-4.5 hour time-window (with an NNT of 10-20), CTP/CTA are key to determining whether the patient requires emergent endovascular thrombectomy in the 0-24 hour time-window (with an NNT of 2.6-4). As these two time-windows overlap, the most practical approach is increasingly to obtain multi-modality imaging up-front / as rapidly as possible in the ED. It is important to remember that as of 2015, both IV tPA and endovascular thrombectomy are considered standard-of-care, and any patient presenting with acute ischemic stroke must undergo full workup and consideration of both treatments based upon national society / consensus guidelines.
An added note on NCCT versus CTP: while NCCT is the oldest modality in the ED, it continues to have tremendous value in acute stroke imaging. Presence or absence of early stroke changes on NCCT (quantified by the ASPECT score) can at times trump CTP in the 0-6 hr time-window, and CTP within any time-window must be interpreted in context of NCCT findings. For example, CTP may show no abnormality (or even luxury perfusion) in an area of established stroke on NCCT in cases of spontaneous recanalization. On the other hand, CTP can be very helpful in detecting small areas of ischemia not well seen on CT/CTA (even when reading NCCT using optimized 35/35 or 40/40 “stroke windows”), and CTP has higher sensitivity for small/distal branch occlusions than either CT/CTA.
The approach to cerebrovascular arterial anatomy is nicely reviewed. A few additional comments:
ICA: acute ICA occlusions are most dramatic when reaching the terminus (thereby blocking the MCA/ACA), but those not reaching the supraclinoid ICA may at times be well-tolerated due to collaterals across the Circle of Willis,
VA: the course/anatomy of the VA is rather variable, with one VA (typically the right) being less dominant as we age; similarly, PICA can have a variable origin and territory of supply, and
BA: while randomized trials of endovascular thrombectomy for basilar occlusion have not been published, the natural history of BA occlusion is typically devastating/fatal, and a large body of non-randomized data (case series/cohorts) shows marked improvement over this natural history following endovascular thrombectomy for BA stroke in selected patients.
Vice Chair of Regional Neurosurgery
Professor of Neurological Surgery
Department of Neurological Surgery
Feinberg School of Medicine
How to Cite this Post
[Peer-Reviewed, Web Publication] Seltzer J, Neill L. (2020, Jan 6). Emergency Guide to Stroke Neuroimaging. [NUEM Blog. Expert Commentary by Jahromi B]. Retrieved from http://www.nuemblog.com/blog/2018/4/20/stroke-neuroimaging
References
National Center for Chronic Disease Prevention and Health Promotion , Division for Heart Disease and Stroke Prevention. “Stroke Fact Sheet.” Last Update: September 1, 2017. Accessed from https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_stroke.htm
Emberson J, Lees KR, Lyden P, et al., for the Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014 Nov 29;384(9958):1929-35.
Filho JO, Samuels OB. Approach to reperfusion therapy for acute ischemic stroke. UpToDate. Last Update: September 14, 2018. Accessed from https://www.uptodate.com/contents/approach-to-reperfusion-therapy-for-acute-ischemic-stroke
Chaela JA, Kidwell CS, Nentwich LM, et al.. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007 Jan 27; 369(9558): 293–298.
Nadgir R, Yousef DM. “Vascular Diseases of the Brain.” In Neuroradiology: The requisites. 4th Ed. (2017). Philadelphia, PA: Mosby/Elsevier
Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004 Apr;35(4):876-81.
Sabarudin A, Subramaniam C, Sun Z. Cerebral CT angiography and CT perfusion in acute stroke detection: a systematic review of diagnostic value. Quant Imaging Med Surg. 2014 Aug;4(4):282-90.
Shen J, Li X, Li Y, Wu B. Comparative accuracy of CT perfusion in diagnosing acute ischemic stroke: A systematic review of 27 trials. PLoS One. 2017 May 17;12(5):e0176622.
Mancall EL. “Vascular Supply of the Brain and Spinal Cord” In Gray's clinical neuroanatomy: The anatomic basis for clinical neuroscience. 1st Ed. (2011). Philadelphia, PA: Elsevier/Saunders.
Mtui E, Gruener G, Dockery P. “Blood Supply of the Brain.” In Fitzgerald’s Clinical Neuroanatomy and Neuroscience. 7th Ed. (2016). Edinburgh: Elsevier Saunders.
Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996 Mar;38(3):425-32.
The Joint Commission. Specifications Manual for Joint Commission National Quality Measures (v2018B). Last Updated: 2018. Accessed from https://manual.jointcommission.org/releases/TJC2018B/DataElem0771.html
Filho JO, Samuels OB. Mechanical thrombectomy for acute ischemic stroke. UpToDate. Last Update: March 22 2019. Accessed from https://www.uptodate.com/contents/mechanical-thrombectomy-for-acute-ischemic-stroke
Prokop M1, Shin HO, Schanz A, Schaefer-Prokop CM. Use of maximum intensity projections in CT angiography: a basic review. Radiographics. 1997 Mar-Apr;17(2):433-51.
Mokin M, Levy EI, Saver JL, Siddiqui AH, Goyal M, Bonafé A, Cognard C, Jahan R, Albers GW; SWIFT PRIME Investigators. Predictive Value of RAPID Assessed Perfusion Thresholds on Final Infarct Volume in SWIFT PRIME (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment). Stroke. 2017 Apr;48(4):932-938.
Lui YW, Tang ER, Allmendinger AM, Spektor V. Evaluation of CT perfusion in the setting of cerebral ischemia: patterns and pitfalls. AJNR Am J Neuroradiol. 2010 Oct;31(9):1552-63.
Bivard A, Levi C, Krishnamurthy V, McElduff P, Miteff F, Spratt NJ, Bateman G, et al.. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain. 2015 Jul;138(Pt 7):1919-31.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B,et al.; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015 Mar 12;372(11):1009-18.
The ED Guide to Neuroimaging: Part 2
Written by: Justin Seltzer, MD (NUEM PGY-3) Edited by: Priyanka Sista, MD, (NUEM PGY-4) Expert commentary by: Peter Pruitt, MD, MS
Make sure to check out The ED Guide to Neuroimaging: Part 1
Part two of this series examines the literature regarding the appropriate use of the head CT in blunt head trauma, a common clinical grey zone in emergency medicine.
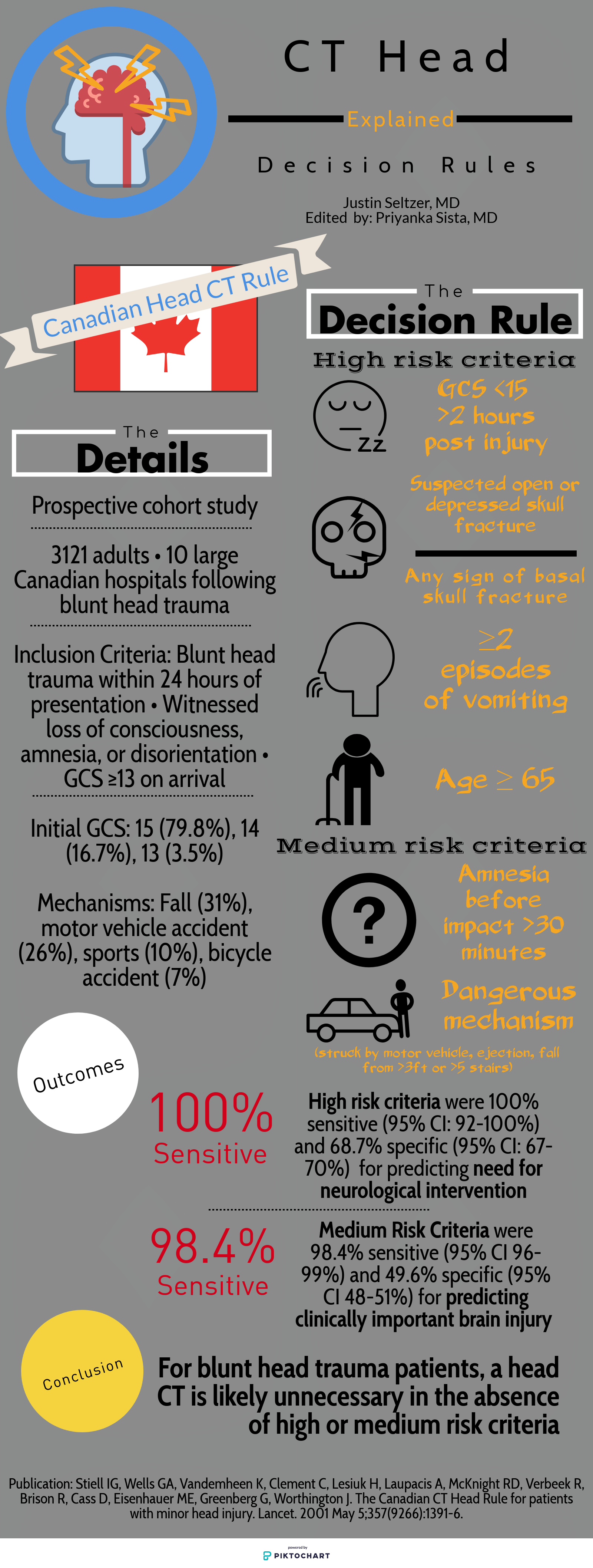
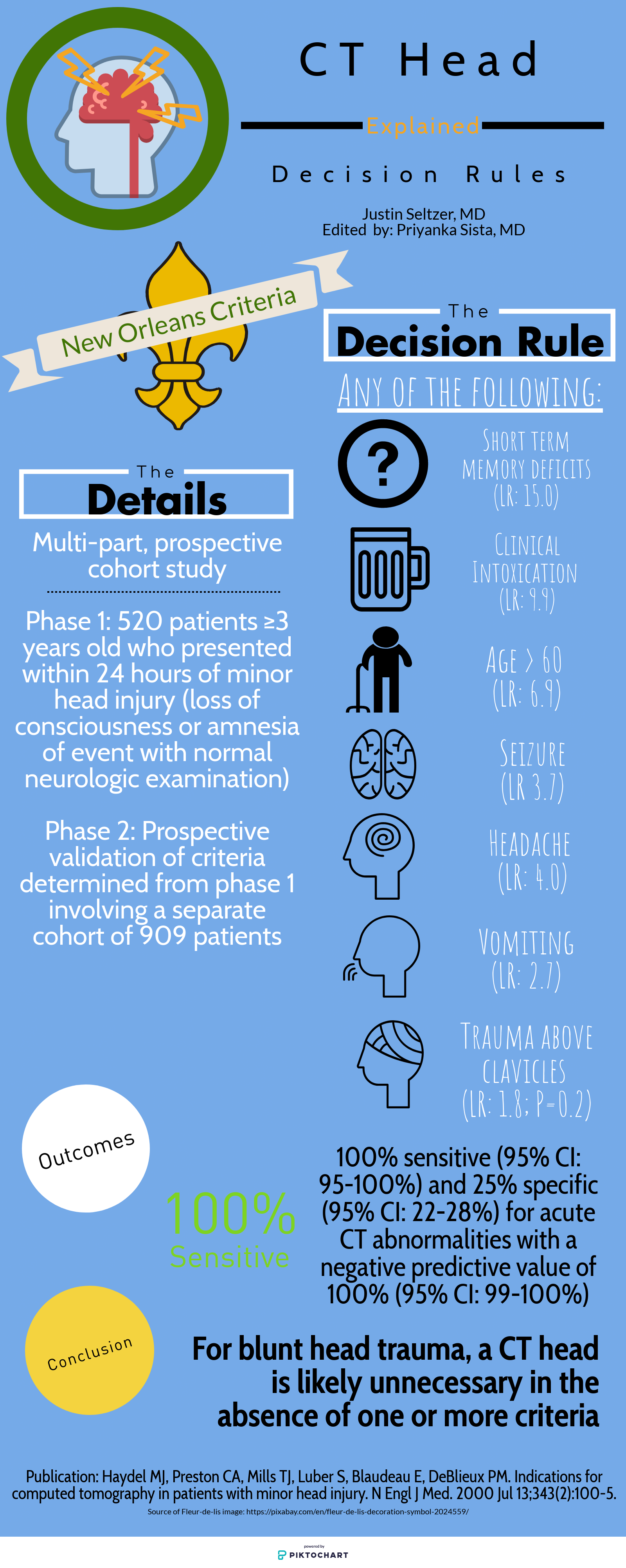
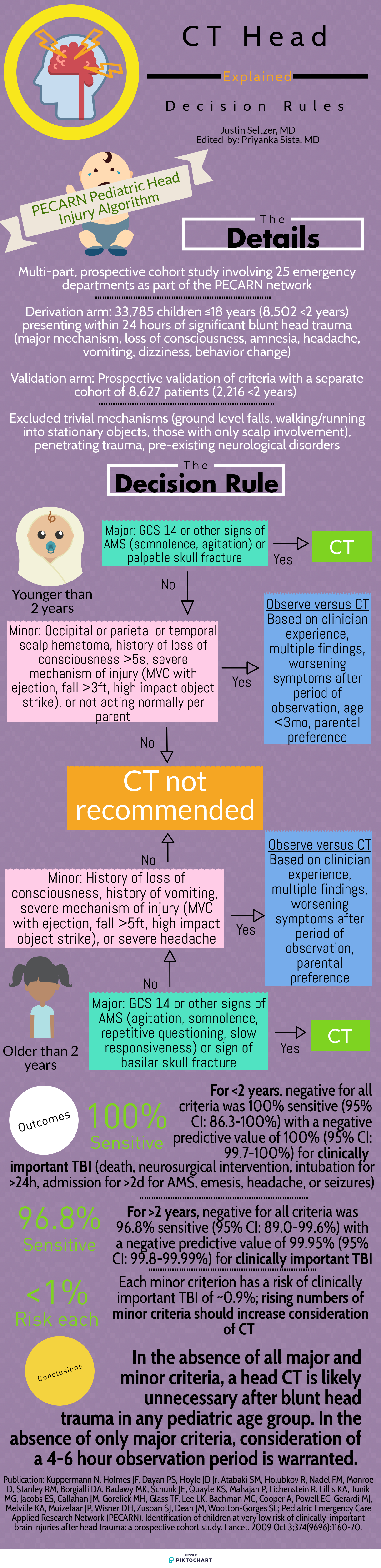
The Canadian Head CT Rule (Canadian), New Orleans Criteria (New Orleans), NEXUS II Head CT Rule (NEXUS), and PECARN Pediatric Head Injury Algorithm (PECARN) are four major decision rules designed to assist clinicians with this often difficult decision. This article is dedicated to comparing these rules and providing a reasonable guide for maximizing their individual utility. The provided infographics detail the specifics of each rule for quick reference.
To start, there are many shared characteristics between the rules. All apply to blunt head trauma only and, except for NEXUS, specifically to those presenting within 24 hours of injury. They utilize criteria to characterize high risk populations for which emergent head CT is appropriate as well as those low risk enough to forego it. Each boasts near perfect sensitivity and negative predictive values for clinically significant acute intracranial processes. Finally, all were prospective cohort studies and, aside from New Orleans, multi-center.
However, there are differences between each rule that can impact their applicability to certain situations and populations.
Study population: The single center New Orleans Criteria had the smallest study population, with 1429 total patients, while the largest, PECARN, had over 42,000 patients. All aside from NEXUS had some age restrictions. Canadian included adults and pediatric patients older than 16 years and New Orleans included adults and pediatric patients older than 3 years. PECARN was exclusively pediatric and excluded anyone over 18 years old.
Inclusion and exclusion criteria: There was significant heterogeneity between the studies on what qualified for inclusion. New Orleans only included patients with known loss of consciousness or post-injury amnesia with a normal neurologic exam. Similarly, Canadian involved patients with GCS ≥13 and witnessed alteration or loss of consciousness. PECARN, on the other hand, was most concerned with mechanism and excluded patients with trivial mechanisms or injuries, such as ground level falls, walking into objects, and isolated scalp involvement. These are further contrasted with NEXUS, which included “all patients with blunt trauma with minor head injury (Glasgow Coma Scale [GCS] score of 15) who present to participating study center.”[1]
Decision rule criteria: Certain criteria, such as evidence of skull fracture, persistent vomiting, older age (>60-65 years), were nearly universally present. However, beyond these there is little consensus. NEXUS, likely because it applies to all ages, includes criteria such as alertness, behavior changes, and scalp hematoma similar to PECARN. Only New Orleans included clinical intoxication, while NEXUS was the only rule to include coagulopathy. Mechanism-based criteria were only considered by Canadian and PECARN.
Primary outcome: There is significant similarity in terms of primary outcome. NEXUS criteria sought “clinically important intracranial injury,” New Orleans any acute abnormality on CT, and PECARN “clinically important traumatic brain injury.” The definitions varied somewhat but were generally similar. Only Canadian stratified differently, with a set of criteria geared towards identifying the need for neurosurgical intervention specifically and another set for the more familiar “clinically important brain injury.”
Methods of Application: NEXUS, Canadian, and New Orleans are all or nothing; meeting even one element results in a head CT and not meeting any means a head CT is likely unnecessary. PECARN is unique in that if the major criteria are not met, minor criteria defer to observation or head CT based in part on non-standardized elements such as physician experience and parental preference. Only in the absence of major and minor criteria can a child be cleared immediately.
Finally, it is important to understand the level of external validation and comparison to which each of these studies has been subjected. Boudia and colleagues performed an external validation study of both Canadian and New Orleans involving 1582 patients 10 years and older over a 3-year period. They noted some key differences between reported performance and performance between the two metrics. Canadian had 100% sensitivity for need for neurosurgical intervention, while New Orleans was 82% sensitive. Canadian was 95% sensitive for clinically significant head CT findings, compared with 86% sensitivity for New Orleans. Negative predictive values were 100% and 99% for Canadian and New Orleans, respectively.[5] Mower, Gupta, Rodriguez, and Hendey recently published a nearly 10 year validation of NEXUS involving 11,770 patients from four centers, which showed improved sensitivity (99% versus 98.3%), specificity (25.6% versus 13.7%), and negative predictive value (99.7% versus 99.1%) compared with the original study for clinically significant intracranial injury. This study also compared NEXUS and Canadian performance within the same study population for those who met Canadian criteria. NEXUS was found to have superior sensitivity (100% versus 97.3%) but worse specificity (32.6% versus 58.8%) for neurosurgical intervention while having worse sensitivity and specificity (97.7%/12.3% versus 98.4%/33.3%) compared with Canadian medium risk criteria for identification of significant brain injury.[6] Schachar and colleagues compared New Orleans, Canadian, and NEXUS in 2,101 pediatric patients over nearly seven years at a non-trauma center; all showed negative predictive values over 97% however Canadian and NEXUS both showed dramatically lower (65.2% and 78.3%, respectively) sensitivities in this population.[7] Smits and colleagues concluded from a Dutch cohort of 3,181 adult patients that Canadian had a lower sensitivity than New Orleans for traumatic intracranial findings but still identified all neurosurgical cases and had a much higher specificity, resulting in a greater number of avoided unnecessary scans.[8] In contrast, PECARN has been externally validated multiple times, all with near perfect sensitivity and negative predictive value;[9-13] of note, in one study two physically abused children with clinically important traumatic brain injury were misclassified as low risk, highlighting a gap in its criteria.[11]
In summary, the four major head CT decision rules all boast impressive sensitivity and negative predictive value for significant traumatic intracranial injury, though external validation and comparison studies have shown that some rules perform better than others under less controlled conditions. When properly applied to the intended patient populations, we can conclude that these are all useful clinical decision making tools, in particular to identify low risk patients and avoid unnecessary radiation exposure, costs, and resource utilization.
Expert Commentary
I applaud Dr. Seltzer for his interesting and informative summary of decision instruments for patients with blunt head trauma. It is important to have a clear strategy for managing patients with this complaint, since traumatic brain injury is one of the most common ED complaints, accounting for an estimated 2.8 million annual visits in 2013, and the number of visits are steadily increasing.[1] Using a well validated decision instrument, such as the Canadian CT Head Rule in adults or the PECARN rule in children, reduces the frequency of unnecessary imaging and decreases length of stay while increasing the diagnostic yield (frequency of positive tests) amongst those patients that are imaged.[2,3] With this in mind, integration of these rules into clinical practice is a key component of appropriate resource utilization, and is recommended by multiple clinical practice guidelines.[4–6] However, the use of decision instruments cannot completely replace clinical gestalt, defined as the impression of the patient derived from the clinical evaluation. Unfortunately, studies comparing decision instruments to gestalt are extremely limited.[7] One study compared the PECARN decision instrument to clinician gestalt and found gestalt to be much more specific with similar sensitivity, although clinicians were asked about the criteria used in the decision instruments prior to making their “gestalt” decision.[8] There are no studies comparing the decision instruments used in adults to gestalt, so their relative performance is still open to assessment. Clinical instinct is still a valuable tool, and decision instruments only function to support this core skill. It is also important to consider what constitutes a positive outcome in these studies. Most notably, the Canadian CT Head Rule in its simplest form does not attempt to identify individuals who will have no hemorrhage at all.[9] Instead, the authors pre-defined defines a “clinically important injury”, which allowed patients to have small subdural hematomas or trace subarachnoid hemorrhage while still being considered low risk by the rule. Because these lesions rarely require intervention, the clinical significance of identifying them is minimal.
Peter Pruitt, MD, MS
Assistant Professor
Department of Emergency Medicine
Northwestern University
How to cite this post
[Peer-Reviewed, Web Publication] Seltzer J, Sista P, (2019, December 15 ). The ED Guide to Neuroimaging: Part 2. [NUEM Blog. Expert Commentary by Pruitt P ]. Retrieved from http://www.nuemblog.com/blog/emergency-neuroimaging-pt2.
Other posts you might enjoy…
References
Mower WR, Hoffman JR, Herbert M, Wolfson AB, Pollack CV Jr, Zucker MI; NEXUS II Investigators. Developing a decision instrument to guide computed tomographic imaging of blunt head injury patients. J Trauma. 2005 Oct;59(4):954-9.
Kuppermann N, Holmes JF, Dayan PS, Hoyle JD Jr, Atabaki SM, Holubkov R, Nadel FM, Monroe D, Stanley RM, Borgialli DA, Badawy MK, Schunk JE, Quayle KS, Mahajan P, Lichenstein R, Lillis KA, Tunik MG, Jacobs ES, Callahan JM, Gorelick MH, Glass TF, Lee LK, Bachman MC, Cooper A, Powell EC, Gerardi MJ, Melville KA, Muizelaar JP, Wisner DH, Zuspan SJ, Dean JM, Wootton-Gorges SL; Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009 Oct 3;374(9696):1160-70.
Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000 Jul 13;343(2):100-5.
Stiell IG, Wells GA, Vandemheen K, Clement C, Lesiuk H, Laupacis A, McKnight RD, Verbeek R, Brison R, Cass D, Eisenhauer ME, Greenberg G, Worthington J. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001 May 5;357(9266):1391-6.
Bouida W, Marghli S, Souissi S, Ksibi H, Methammem M, Haguiga H, Khedher S, Boubaker H, Beltaief K, Grissa MH, Trimech MN, Kerkeni W, Chebili N, Halila I, Rejeb I, Boukef R, Rekik N, Bouhaja B, Letaief M, Nouira S. Prediction value of the Canadian CT head rule and New Orleans for positive head CT scan and acute neurosurgical procedures in minor head trauma: a multicenter external validation study. Ann Emerg Med. 2013 May;61(5):521-7.
Mower WR, Gupta M, Rodriguez R, Hendey GW. Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) Head computed tomographic (CT) decision instrument for selective imaging of blunt head injury patients: An observational study. PLoS Med. 2017 Jul 11;14(7):e1002313.
Schachar JL, Zampolin RL, Miller TS, Farinhas JM, Freeman K, Taragin BH. External validation of New Orleans (NOC), the Canadian CT Head Rule (CCHR) and the National Emergency X-Radiography Utilization Study II (NEXUS II) for CT scanning in pediatric patients with minor head injury in a non-trauma center. Pediatr Radiol. 2011 Aug;41(8):971-9.
Smits M, Dippel DW, de Haan GG, Dekker HM, Vos PE, Kool DR, Nederkoorn PJ, Hofman PA, Twijnstra A, Tanghe HL, Hunink MG. External validation of the Canadian CT Head Rule and New Orleans for CT scanning in patients with minor head injury. JAMA. 2005 Sep 28;294(12):1519-25.
Schonfeld D, Bressan S, Da Dalt L, Henien MN, Winnett JA, Nigrovic LE. Pediatric Emergency Care Applied Research Network head injury clinical prediction rules are reliable in practice. Arch Dis Child. 2014 May;99(5):427-31.
Lorton F, Poullaouec C, Legallais E, Simon-Pimmel J, Chêne MA, Leroy H, Roy M, Launay E, Gras-Le Guen C. Validation of the PECARN clinical decision rule for children with minor head trauma: a French multicenter prospective study. Scand J Trauma Resusc Emerg Med. 2016 Aug 4;24:98.
Ide K, Uematsu S, Tetsuhara K, Yoshimura S, Kato T, Kobayashi T. External Validation of the PECARN Head Trauma Prediction Rules in Japan. Acad Emerg Med. 2017 Mar;24(3):308-314.
Babl FE, Borland ML, Phillips N, Kochar A, Dalton S, McCaskill M, Cheek JA, Gilhotra Y, Furyk J, Neutze J, Lyttle MD, Bressan S, Donath S, Molesworth C, Jachno K, Ward B, Williams A, Baylis A, Crowe L, Oakley E, Dalziel SR; Paediatric Research in Emergency Departments International Collaborative (PREDICT). Accuracy of PECARN, CATCH, and CHALICE head injury decision rules in children: a prospective cohort study. Lancet. 2017 Jun 17;389(10087):2393-2402.
Nakhjavan-Shahraki B, Yousefifard M, Hajighanbari MJ, Oraii A, Safari S, Hosseini M. Pediatric Emergency Care Applied Research Network (PECARN) prediction rules in identifying high risk children with mild traumatic brain injury. Eur J Trauma Emerg Surg. 2017 Dec;43(6):755-762.
References (Expert Commentary)
Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths — United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1-16.
Sharp AL, Huang BZ, Tang T, et al. Implementation of the Canadian CT Head Rule and Its Association With Use of Computed Tomography Among Patients With Head Injury. Ann Emerg Med. 2017;33(0):1505-1514.
Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294(12):1511-1518.
Rosenberg A, Agiro A, Gottlieb M, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. October 2015:1.
Schuur JD, Carney DP, Lyn ET, et al. A top-five list for emergency medicine a pilot project to improve the value of emergency care. JAMA Intern Med. 2014;174(4):509-515.
Mills AM, Raja AS, Marin JR. Optimizing Diagnostic Imaging in the Emergency Department. Acad Emerg Med. 2015:n/a-n/a.
Schriger DL, Elder JW, Cooper RJ. Structured Clinical Decision Aids Are Seldom Compared With Subjective Physician Judgment, and are Seldom Superior. Ann Emerg Med. 2016.
Babl FE, Oakley E, Dalziel SR, et al. Accuracy of Clinician Practice Compared With Three Head Injury Decision Rules in Children: A Prospective Cohort Study. Ann Emerg Med. 2018;71(6):703-710.
Stiell IG, Lesiuk H, Wells GA, et al. The Canadian CT head rule study for patients with minor head injury: Rationale, objectives, and methodology for phase I (derivation). Ann Emerg Med. 2001;38(2):160-169.
Rise and Shine: A Review of the WAKE-UP Trial
Written by: Gabrielle Bunney, MD (NUEM PGY-2) Edited by: Alex Ireland, (NUEM PGY-4) Expert commentary by: Chris Richards, MD, MS
Introduction
Wake up strokes have always been a clinical conundrum. Current practice guidelines from the American Stroke Association on the treatment of acute ischemic strokes specify a maximum of 4.5 hours from time of symptom onset to the delivery of alteplase therapy. [1] However, patients often awaken with these symptoms or are unable to give a clear history of symptom onset and thus are not eligible for alteplase therapy. Initial non-contrast computed tomography can identify whether or not an acute hemorrhage is present, but confirmatory imaging for ischemic stroke involves magnetic resonance imaging (MRI). Studies are now looking at the utility of early MRI in the diagnostic and therapeutic pathways of acute ischemic stroke. These studies are specifically looking at a positive signal on diffusion-weighted imaging (DWI) and a negative signal on FLAIR imaging to identify recent cerebral infarction. Multiple studies have found that there is adequate sensitivity and specificity of DWI-FLAIR mismatch to suggest stroke onset within 4.5 hours. [2-4] Armed with these new data, this paper’s goal was to determine whether patients with an unknown time of symptom onset, but with a mismatch on DWI and FLAIR MRI imaging, would benefit from thrombolysis with intravenous alteplase.
Study
Thomalla G, Simonsen CZ, et. al “MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset | NEJM.” New England Journal of Medicine, Oxford University Press, www.nejm.org/doi/full/10.1056/NEJMoa1804355. [5]
Study Design
This study was a multi-center, randomized, double blind, and placebo controlled clinical trial. It involved 70 experienced stroke research centers in eight European countries. There was a central image-reading committee that reviewed all images for patient enrollment to evaluate inclusion and exclusion, and to arbitrate disagreements.
Population
Patients between the ages of 18 and 80 were eligible for the study if they clinically had an acute stroke and were able to perform their activities of daily living prior to this event. The patient had to awaken with these symptoms, be unsure about the time of onset secondary to confusion or aphasia, or have a timeline of symptoms greater than 4.5 hours, without an upper limit. 1,362 patients underwent screening. 859 were excluded, leaving 503 that were randomized. 254 of those were given alteplase and 249 received placebo.
Intervention Protocol
Selected patients underwent DWI and FLAIR MRI imaging. Those who had a mismatch, defined as an abnormal signal on DWI, but no signal on FLAIR, were then randomized. Excluded were those with intracranial hemorrhage, lesions larger than one third of the middle cerebral artery territory, those who were to undergo thrombectomy, those with severe stroke, defined as greater than 25 on the National Institute of Health Stroke Scale (NIHSS), and those that had any other contraindication to alteplase aside from time from last known normal. Those that were randomized into the alteplase group were given 0.9mg/kg of alteplase with 10% administered as a bolus and the rest given as an infusion over 60 minutes. Assessments were then conducted between 22 and 36 hours after randomization, between 5 and 9 days, and finally at 90 days.
Outcome Measures
This study had two end point measurements: efficacy and safety. The primary efficacy outcome measurement was favorable clinical outcome defined as a score of 0 to 1 on the modified Rankin scale 90 days after randomization. Secondary efficacy outcome measurements ranged from depression scores to activities of daily living measurements.
The primary safety outcome was death and a composite outcome of death or dependence (4-6 on the modified Rankin scale) at 90 days. Secondary safety endpoints were symptomatic intracranial hemorrhage and the incidence of parenchymal hematoma type 2 on MRI 22 to 36 hours after randomization.
Results
The demographics between the alteplase and placebo groups were similar for age, sex, and medical history. However, in the alteplase group there was a higher rate of intracranial occlusion of the internal carotid artery. The average time for treatment of the alteplase and placebo groups was 3.1 and 3.2 hours after symptom recognition, respectively.
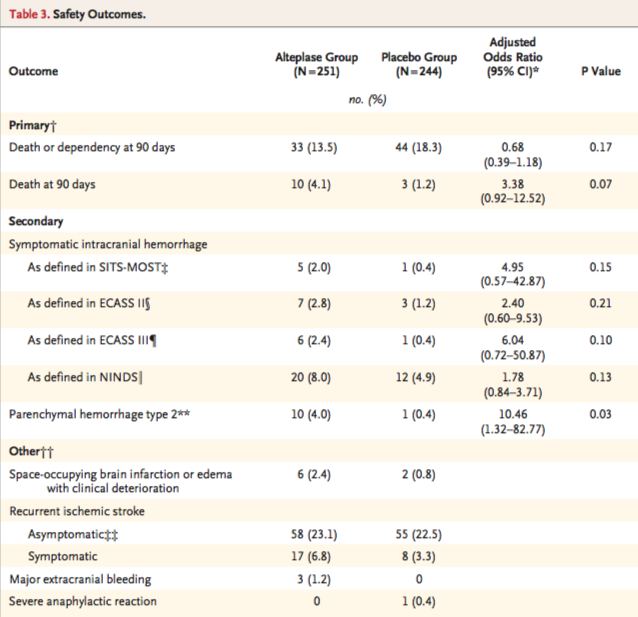
Alteplase was found to be associated with favorable outcome at 90 days, with 53.3% in the alteplase group and only 41.8% in the placebo group having a modified Rankin score between 0 and 1 at 90 days, p=0.02. The secondary efficacy endpoints lacked power due to the fact that the study was terminated early because of a loss of funding. Table 2 from the original article describes the efficacy findings.
The safety groups were sized 251 in the alteplase group due to 5 patients not receiving alteplase and 244 in the placebo group due to 4 patients not receiving placebo. Death or dependency was found in 13.5% of the alteplase group and 18.3% of the placebo group, p=0.17. However, death at 90 days was higher in the alteplase group at 4.1%, while in the placebo group it was 1.2%, p=0.07. There were numerically more parenchymal hemorrhages in the alteplase group than in the placebo group, with 10 in the alteplase group and 1 in the placebo group. Table 3 from the original article describes the safety outcomes.
Interpretation
The primary efficacy outcome of this study, favorable functional outcome at 90 days, was higher in the group that received alteplase than in the group that received placebo and was statistically significant. Additionally, the primary safety outcome of death or disability was higher in the placebo group than in the alteplase group, though this was not found to be statistically significant. Death at 90 days was found to be numerically higher in the alteplase group than the placebo group, although not statistically significant. In extrapolating the data from the paper, the number needed to treat is 9.4 and the number needed to harm is 36.3. The ratio of these numbers suggests that treatment provides a greater benefit than risk.
There are several limitations to this study. The trial was stopped early due to lack of funding, and so we may be overestimating the benefit or underestimating the risk. The authors estimated that they needed approximately 800 patients to have sufficient power, yet enrolled only 503. Bleeding complications and death at 90 days were numerically higher in the alteplase group, though this was not statistically significant. Trials such as ATLANTIS A and B, and ASK, were all stopped early due to harm because of increased bleeding in the alteplase groups. [6-8] It is unknown whether the addition of 297 patients to meet the pre-specified enrollment target of 800 in the WAKE-UP trial would have resulted in statistical significance.
The population of this study had a median NIHSS of 6 out of 42, a relatively low stroke severity. The DAWN, DEFUSE, NINDS, and SITS-MOST trials, all significant studies in the progression of stroke research, had NIHSS medians of 17, 16, 14, and 12, respectively. [9-12] It is unclear if MRI-guided alteplase therapy would benefit patients with more severe strokes. Additionally, this paper excluded patients who were selected for thrombectomy. Patients selected for thrombectomy have large clot burdens in the internal carotid artery or middle cerebral artery that often have a modified Rankin score greater than 6. [13] By not including these patients, the WAKE-UP trial does not show the benefit of medical treatment in these sicker patients with a larger clot burden.
Lastly, the study was only performed in experienced research stroke centers with readily available diagnostic pathways and MRI. Of the 1,362 patients imaged and screened, only 37% met intervention criteria. Many did not have DWI-FLAIR mismatch, and some did not have any DWI lesion, suggesting a transient ischemic attack or a stroke mimic. A smaller hospital is unlikely to have the experience or equipment to be able to screen these more difficult patients for the few that can actually proceed to intervention.
Future Areas of Research
A replication of this study with additional subjects and sufficient power to confirm the beneficial effect of alteplase in MRI-guided thrombolysis would be the next step. Inclusion of alteplase plus thrombectomy in appropriate patients presenting after 4.5 hours with mismatch on DWI-FLAIR is another possible study. For example, TWIST (ClinicalTrials.gov Identifier: NCT03181360) and TIMELESS (ClinicalTrials.gov Identifier: NCT03785678) are two large clinical trials that will hopefully give more information about expanding the time window for thrombolysis. [14,15]
Review
MRI DWI-FLAIR mismatch may be able to allow more patients to receive alteplase therapy after an acute ischemic stroke
Alteplase still shows benefit for treating stroke even with an unknown timeline when used in conjunction with MRI DWI-FLAIR mismatch
Similar to prior studies, alteplase is associated with numerically higher instances of intracranial hemorrhage
Further research needs to be done to increase the power of this study
Expert Commentary
Really nice summary of the recent WAKE-UP* trial, and you bring up important considerations about both the pros and the cons of this study. WAKE-UP addressed an important clinical question: is it safe and effective for patients who have no other disqualifying reasons aside from their last known normal time to receive thrombolysis, if imaging shows a small area of infarction but a large area of ischemia? As you mention, intervention patients in the study: a) received intravenous tissue plasminogen activator (IV tPA) when otherwise they would not have, b) had increased odds of having a favorable outcome compared to standard of care, c) though with numerically greater instances of hemorrhage.
WAKE-UP fits into a narrative with two other important recent trials, DAWN* and DEFUSE-3*that studied the outcomes of patients with last known normal (LKN) times greater than conventional LKN time cut-offs (4.5 hours for IV tPA and 6 hours for endovascular therapy) and have found efficacy of reperfusion in these extended windows for select patients. A third trial, EXTEND,* has been presented in abstract form and has demonstrated clinical improvement in select AIS patients receiving IV tPA up to 9 hours from LKN time (https://abstractsonline.com/pp8/#!/4715/presentation/13367). Importantly, these trials that expand the time window for reperfusion used imaging-based criteria for inclusion: WAKE-UP used MR, DAWN used a non-contrast CT scan compared to severity of clinical syndrome, DEFUSE-3 used CT perfusion along with a computer software program to identify the infarcted “core” and the ischemic penumbra, and EXTEND required a penumbral mismatch on CT perfusion or MRI.
From a pathophysiological perspective, this makes sense. If imaging can identify a large area of ischemia and small area of infarction, reperfusion should potentially result in the salvage of at least some of those reversibly damaged cells. It should also result in less pronounced hemorrhagic side effects because the area of known infarction is less – remember, not only neurons die in infarcted brain, so do blood vessel endothelium cells, a contributing factor in post-reperfusion hemorrhage. [16]
Looking into the future of acute stroke care, these clinical trials give promise for individualized acute stroke treatment. Rather than being beholden to a rigid time cut-off (that evidence is showing is not one-size-fits-all), we can look to imaging to inform acute treatment decision. We have learned from subgroup analysis from DEFUSE-3 that some patients slowly progress in their stroke pathophysiology, meaning that even beyond 24 hours, some patients have a favorable core to penumbra ratio. [17] Other patients quickly progress in their stroke pathophysiology and may match their ischemic core to their salvageable penumbra well before traditional time-cut offs. [18]
It is possible that image-based selection criteria could be integral to the screening of all candidates for acute reperfusion therapy in the future. As WAKE-UP, EXTEND, DAWN, and DEFUSE-3 have shown us, there are some patients that can be reasonably considered for treatment beyond traditional time cut-offs. The same imaging criteria that extended the window for patients in these studies may be the same criteria that, in the future, could identify patients within the traditional time window who are likely to not benefit from treatment and who may have an increased risk of hemorrhagic conversion. One can image a patient without evidence of a salvageable penumbra presenting at 3 hours, for example, for whom the risks of IV tPA may, in fact, outweigh the potential benefits.
Lastly, I would hazard readers from interpreting the results of WAKE-UP, EXTEND, DAWN, and DEFUSE-3 as providing comfort in delaying thrombolysis or endovascular therapy for patients in extended time windows who would otherwise have indications for reperfusion. For IV tPA, longer delay is associated with increased risk of symptomatic intracranial hemorrhage and more timely treatment is associated with better outcomes. In the 2015 endovascular therapy trials, [19-23] even for patients within 6 hours of LKN, more timely treatment was associated with better outcomes. Even if the imaging protocols used in WAKE-UP, EXTEND, DAWN, and DEFUSE-3 can identify “slow progressors” that can be treated outside current treatment windows, these patients’ stroke are still progressing as time goes by. [18] Systems that promote timely evaluation of patients with stroke systems should be expected to help patients in extended time window, as they do patients within traditional time windows. {24,25]
Studies like WAKE-UP that test traditional inclusion and exclusion criteria for reperfusion give promise for safer and more effective stroke treatment. We look forward to future clinical trials, like TIMELESS* and TWIST*, that hopefully will give further clarity on this clinical question.
WAKE-UP [5]: Efficacy and Safety of MRI-based Thrombolysis in Wake-up Stroke Trial
DAWN [10]: Diffusion Weighted Imaging (DWI) or Computerized Tomography Perfusion (CTP) Assessment With Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention Trial
DEFUSE-3 [9]: Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 Trial
EXTEND [26]: EXtending the time for Thrombolysis in Emergency Neurological Deficits Trial
TIMELESS: Tenecteplase in Stroke Patients Between 4 and 24 Hours Trial (https://clinicaltrials.gov/ct2/show/NCT03785678)
TWIST: Tenecteplase in Wake-up Ischaemic Stroke Trial (https://clinicaltrials.gov/ct2/show/NCT03181360)
Chris Richards, MD, MS
Assistant Professor
Department of Emergency Medicine
Northwestern University
How to Cite this Post
[Peer-Reviewed, Web Publication] Bunney G, Ireland A. (2019, Sept 9). Rise and Shine: A Review of the WAKE-UP Trial. [NUEM Blog. Expert Commentary by Richards C]. Retrieved from http://www.nuemblog.com/blog/wake-up-trial.
Other Posts You May Enjoy
Citations
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018;49:e46-e110.
Aoki, Junya, et al. “FLAIR Can Estimate the Onset Time in Acute Ischemic Stroke Patients.” Journal of the Neurological Sciences, vol. 293, no. 1-2, 2010, pp. 39–44., doi:10.1016/j.jns.2010.03.011
Petkova, Mina, et al. “MR Imaging Helps Predict Time from Symptom Onset in Patients with Acute Stroke: Implications for Patients with Unknown Onset Time.” Radiology, vol. 257, no. 3, 2010, pp. 782–792., doi:10.1148/radiol.10100461.
Thomalla, Götz, et al. “DWI-FLAIR Mismatch for the Identification of Patients with Acute Ischaemic Stroke within 4·5 h of Symptom Onset (PRE-FLAIR): a Multicentre Observational Study.” The Lancet Neurology, vol. 10, no. 11, 2011, pp. 978–986., doi:10.1016/s1474-4422(11)70192-2.
Thomalla G, Simonsen CZ, et. al “MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset | NEJM.” New England Journal of Medicine, Oxford University Press, www.nejm.org/doi/full/10.1056/NEJMoa1804355.
Albers, Gregory W., et al. “ATLANTIS Trial.” Stroke, vol. 33, no. 2, 2002, pp. 493–496., doi:10.1161/hs0202.102599.
Clark, Wayne M., et al. “Recombinant Tissue-Type Plasminogen Activator (Alteplase) for Ischemic Stroke 3 to 5 Hours After Symptom Onset.” Jama, vol. 282, no. 21, Jan. 1999, p. 2019., doi:10.1001/jama.282.21.2019.
Donnan, G. A. “Streptokinase for Acute Ischemic Stroke with Relationship to Time of Administration: Australian Streptokinase (ASK) Trial Study Group.” JAMA: The Journal of the American Medical Association, vol. 276, no. 12, 1996, pp. 961–966., doi:10.1001/jama.276.12.961.
Albers, Gregory W, et al. “Thrombectomy for Stroke with Selection by Perfusion Imaging.” New England Journal of Medicine, vol. 378, no. 19, 2018, pp. 1849–1850., doi:10.1056/nejmc1803856.
Nogueira, Raul G, et al. “Thrombectomy 6 to 24 Hours after Stroke.” New England Journal of Medicine, vol. 378, no. 12, 2018, pp. 1161–1162., doi:10.1056/nejmc1801530.
“Tissue Plasminogen Activator for Acute Ischemic Stroke.” New England Journal of Medicine, vol. 333, no. 24, 1995, pp. 1581–1588., doi:10.1056/nejm199512143332401.
Wahlgren, Nils, et al. “Thrombolysis with Alteplase for Acute Ischaemic Stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an Observational Study.” The Lancet, vol. 369, no. 9558, 2007, pp. 275–282., doi:10.1016/s0140-6736(07)60149-4.
Campbell, Bruce C V, et al. “Endovascular Thrombectomy for Stroke: Current Best Practice and Future Goals.” Bmj, vol. 1, no. 1, 2016, pp. 16–22., doi:10.1136/svn-2015-000004.
“Tenecteplase in Wake-up Ischaemic Stroke Trial (TWIST).” ClinicalTrials.gov, clinicaltrials.gov/ct2/show/NCT03181360.
“Tenecteplase in Stroke Patients Between 4 and 24 Hours (TIMELESS).” ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03785678
Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA neurology 2014;71:1181-5. PMCID: 4592535.
Christensen S, Mlynash M, Kemp S, et al. Persistent Target Mismatch Profile >24 Hours After Stroke Onset in DEFUSE 3. Stroke 2019;50:754-7. PMCID.
Rocha M, Jovin TG. Fast Versus Slow Progressors of Infarct Growth in Large Vessel Occlusion Stroke: Clinical and Research Implications. Stroke 2017;48:2621-7. PMCID.
Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11-20. PMCID.
Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009-18. PMCID.
Fransen PS, Berkhemer OA, Lingsma HF, et al. Time to Reperfusion and Treatment Effect for Acute Ischemic Stroke: A Randomized Clinical Trial. JAMA neurology 2015:1-7. PMCID.
Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30. PMCID.
Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-306. PMCID.
Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA 2015;313:1451-62. PMCID.
Higashida R, Alberts MJ, Alexander DN, et al. Interactions within stroke systems of care: a policy statement from the American Heart Association/American Stroke Association. Stroke 2013;44:2961-84. PMCID.
Churilov L, Ma H, Campbell BC, Davis SM, Donnan GA. Statistical Analysis Plan for EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) trial. International journal of stroke : official journal of the International Stroke Society 2018:1747493018816101. PMCID.
The Seriousness of Deliriousness: Delirium in the ED
Written by: Nery Porras, MD (NUEM PGY-2) Edited by: Katie Colton, MD (NUEM PGY-4) Expert commentary by: Lee Lindquist, MD
Introduction:
Imagine: you arrive to your shift, surrounded by the beeping and buzzing of monitors, an intoxicated patient already on the bed in front of your computer. You gamely walk in to the room of your first patient, a 74 year-old man for whom the tracking board reads “something is not right.” You see a quiet, subdued elderly man who looks at first glance generally well. The family member in the room starts talking immediately, concerned that something is just not right with this man – he seems depressed, and has been forgetting names. As she talks you hear a commotion from the next room and poke your head around the divider to see if you can help. There you see an older woman fighting with EMS and her EKG leads, surrounded by nurses trying to keep her from jumping off the stretcher. These widely disparate presentations potentially represent ends of the spectrum of the important but often missed diagnosis of acute delirium.
Emergency physicians are trained in the diagnosis and initial management of life and limb-threatening medical conditions. Delirium is an under-recognized but highly morbid condition that we are under-prepared to diagnose and treat in a busy ED. Trained in pattern recognition, we currently lack an easy and reliable method to diagnose acute delirium. The DSM-V identifies 5 key features that characterize this diagnosis:
Disturbance in attention and awareness
Develops over a short period of time, represents a change from baseline, and tends to fluctuate during the course of the day
An additional disturbance in cognition
The disturbances are not better explained by another preexisting, evolving or established neurocognitive disorder
There is evidence that the disturbance is caused by a medical condition, substance intoxication or withdrawal, or medication side effect
What’s the big deal with a somewhat confused elderly patients?
As the US population ages, elderly Americans are becoming the predominant users of healthcare with over 20 million older American visits to the ED each year (Han, et al 2013); these older patients are preferentially affected by delirium. One study notes delirium to be present in 8-17% of all elderly ED patients and in 40% of nursing home patients (Inouye 2014). Acute delirium can be the presentation of potentially life-threatening conditions including metabolic derangements, sepsis, and hypoxia. Interestingly one study showed that patients in their eighties with myocardial infarction are more likely to present with delirium than chest pain (Inouye 2014).
The diagnosis of delirium itself confers morbidity beyond the underlying condition that it can reflect. When comparing groups of elderly patients in the ED, those with delirium were found to be markedly more likely to die within 6 months than those without delirium – 36% versus 10%, respectively (Han 2013). Another study found that elderly patients diagnosed with delirium had 12-month mortality of 10-26%, rates comparable to sepsis or acute MI (Gower 2012). Despite the remarkable danger of this condition, emergency physicians are bad at making this diagnosis – per one report missing 57-83% of cases of delirium (Han 2013).
What does a patient with delirium look like?
The fluctuating presentation of delirium makes it difficult to recognize but we should be attentive to certain hallmarks, including alterations in attention and awareness and acute changes in cognition. These can be associated with hallucinations or other perceptual disturbances. Collateral information and family input can be critical in detecting changes from baseline function and cognition. The more acute temporal course of delirium is important to distinguish from underlying dementia, which is itself one of the most important risk factors for delirium. The most common presentation, the hypoactive form, is a quiet, subdued, withdrawn state. This is distinguished from the hyperactive form notable for hypervigilance, irritability and restlessness. Hypoactive and mixed-type deliriums are the most common presentations in the ED, representing up to 96% of acute cases in the ED (Han 2013). Unfortunately, the hypoactive form is both more commonly missed and associated with a worse prognosis (Inouye 2014).
So, how do I diagnose delirium? Can this be done in the ED?
As the personal, medical, and financial risks of delirium are increasingly recognized, research has focused on creating better tools for recognition and diagnosis. Traditionally this diagnosis has required an extended interview and evaluation, usually performed by a psychiatrist or geriatrician; these methods are rarely available in the ED or inpatient setting. Inouye and her colleagues built upon the DSM definition of delirium to create the Confusion Assessment Method (CAM) tool to quickly and easily screen for delirium.
The CAM algorithm for diagnosis of delirium, using standardized psychiatric evaluation for delirium for validation, showed a sensitivity of 94%-100% and a specificity of 90%-95% for the diagnosis of delirium (Inouye 1990). The CAM was later adapted and validated for use in the ED (Lewis 1995) and most recently that CAM has been modified to be even more ED friendly. In 2013 the Geriatric Emergency Department Guidelines were put together by ACEP, SAEM, AGS (American Geriatric Society) and ENA (Emergency Nurses Association in which they delineate a real-world adaptation of the CAM, the bCAM. In conjunction with the Delirium Triage Screen, these two tools are quick, easy and practical method of assessing for delirium in the ED.
What should be in your differential diagnosis when you suspect delirium?
You have made the step of recognizing that your elderly patient is off their baseline; what should you consider as part of your work-up? Consider the acronym “I WATCH DEATH” (below), a helpful reminder of the broad (and overlapping) differential of diagnoses that exist for acute delirium. For a more nimble tool particularly useful in the ED evaluation of the delirious elderly patient, the “ABCDEF” acronym (below) was developed by Rosen et al (Rosen 2016) to identify overlooked precipitating causes.
What can I do to acutely de-escalate a delirious patient?
As always, consider non-invasive measures first – recruiting family for this can be critical. Alleviating pain and discomfort and frequent re-orientation can help calm an acutely agitated patient.
Pharmacological interventions should be reserved for the agitated patient with overt psychotic manifestations. The use of restraints should be avoided if possible as the use of restraints themselves can worsen delirium. If medications are required, low doses of haloperidol of 0.5-1 mg can be used, although caution must be exercised with regard to QTc prolongation in patients with polypharmacy (Gower 2012). Atypical antipsychotics can be considered as well; the chart below details one potential protocol for pharmacologic management of acute delirium.
Take Home Points:
Delirium is under-recognized and under-diagnosed but carries marked increases in morbidity and mortality in the elderly population.
This diagnosis can and should be made in the ED; consider diagnostics aids like the CAM.
Delirium is often the presenting symptom of an underlying pathology and a broad differential should be consider.
Pharmacological interventions are a last resort; if needed low does of haloperidol or other atypical antipsychotics can be considered.
Expert Commentary
I love that delirium in the emergency department is being discussed! As a geriatrician, so many of my older adult patients are brought into the ED with these symptoms and it is vital that delirium is recognized and treated effectively. Delirium is a serious problem among older adults – and their families and supporters. It carries a high risk of extended hospitalizations, morbidity, and mortality. Katie and Nery did an excellent job describing the full spectrum of Delirium – ranging from hypo to hyperactive delirium. The hypoactive delirium symptoms are frequently missed because older adults affected by this are very quiet and often appear distracted. With “the squeaky wheel getting the grease”, we are more likely to recognize and respond to the older adult with hyperactive delirium instead.
I fully concur that collateral information is important. I would emphasize that we need to encourage patient families and supporters to stay with the patient – even after the patient is being admitted and moved to the inpatient setting. What can be most frustrating is that families will sometimes leave once the patient is called from the waiting room or after they have been seen by one staff member. We need to reaffirm with families that they need to remain at bedside and continue to remain at bedside when the patient is admitted. Having a loved one nearby will help both physicians in obtaining more information as well as the patient is reorienting and soothing them. I have seen too often that family members will leave from the ED, since the patient is being admitted, and subsequently, the patient’s agitation worsens. Important lesson: Please tell families to follow the patients to the inpatient floors/rooms and continue to stay with them.
Besides the family, collateral information should be obtained from the patient’s prior residence. So many older adult patients are transferred from skilled nursing facilities (SNF), or senior residences/communities (e.g. assisted living, memory care) for worsening confusion and delirium. I would suggest that ED teams contact the SNF nurses to determine collateral information. It would also be helpful to become familiar with the places that house seniors in their area (e.g. set up a tour, ask for clinical/nurse station phone numbers) so that communication can flow more easily.
From a clinical practice, the most common causes of delirium that I see are medication-related (withdrawal, polypharmacy) and dehydration. Doing a medication/brown bag review is always my first step. What I see is that older adults will sometimes start an OTC medication (e.g. diphenhydramine, sleep aid, or a pain medicine/sleep aid combo like a “acetaminophen PM”) and that will cause the acute confusion. Dehydration is also a common cause for delirium among older adults since we lose our thirst mechanism as we age and do not feel the need to drink. With summer weather, it is easy for a senior to become dehydrated with the first symptoms being delirium.
I would like to point out that UTIs are frequently misdiagnosed as a cause for delirium. What does that mean? We have been taught that UTIs can be the culprit for delirium - but it is usually not. Actually, checking a UA for confusion alone is no longer recommended by the CDC. The CDC recommends that urinary symptoms (e.g. frequency, dysuria) are present for UA testing. Why? Many older adults have asymptomatic bacteriuria – so much so that UTIs are frequently over-diagnosed in the ED as the culprit for delirium. When the culture comes back days later, there is a lack of evidence for the UTI. Even on the graphic presented by Katie and Nery on infections causing delirium – UTI is not listed. What I will do is ask for collateral information about any prior urinary symptoms from the families, fevers/chills, leukocystosis, and check for suprapubic tenderness on exam to assess urinary issues as a culprit for delirium.
Lastly, avoid benzodiazepines to treat delirium in older adults. I realize that it is listed on the infographic but this class of medicines are likely to exacerbate the delirium. Benzos can be used for alcohol withdrawal symptoms/delirium tremens but that is it. The ABIM Choosing Wisely Campaign has noted this “Don’t use benzodiazepines or other sedative-hypnotics in older adults as first choice for insomnia, agitation or delirium.” Non-pharmacologic methods are by far the best to treat delirium.
Delirium is not fun for our older adults or their families. The quicker we can recognize the symptoms of delirium and ameliorate the cause in the ED, the better everyone’s lives are. I congratulate Nery and Katie for a great discussion of delirium and I hope that it will impact how we treat our seniors with delirium!
Resources:
ABIM Choosing Wisely – Geriatrics. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/
Mody L, Juthani-Mehta M: Urinary tract infections in older women: a clinical review. Jama 2014, 311(8):844-854.
McKenzie R, Stewart MT, Bellantoni MF, Finucane TE: Bacteriuria in individuals who become delirious. Am J Med 2014, 127(4):255-257.
Nicolle L.E., Bradley S., Colgan R., Rice J.C., Schaeffer A., Hooton T.M. Infectious Diseases Society of America Guideline for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin. Infect. Dis. 2005;40:643–654.
Lee Lindquist, MD, MPH
Associate Professor of Medicine
General Internal Medicine and Geriatrics
How To Cite This Post
[Peer-Reviewed, Web Publication] Porras N, Lindquist L. (2019, March 25). The seriousness of deliriousness: delirium in the ED [NUEM Blog. Expert Commentary by Lindquist L]. Retrieved from http://www.nuemblog.com/blog/delirium
Other Posts You May Enjoy
Resources
Gower, Lynn, et al. “Emergency Department Management of Delirium in the Elderly.” Western Journal of Emergency Medicine, vol. 13, no. 2 Jan. 2013, pp. 194-201.
Grossmann, Florian F. et al. “Screening, detection and management of delirium in the emergency department – a pilot study on the feasibility of a new algorithm for use in older emergency department patients: the modified Confusion Assessment Method for the Emergency Department (MCAM-ED).” Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, vol. 22, no. 1, 2014, p. 19.
Geriatric Emergency Department Guidelines. (2014). Annals of Emergency Medicine, 63(5). doi:10.1016/j.annemergmed.2014.02.008
Han, Jin H., et al. “Delirium in the Older Emergency Department Patient: A Quiet Epidemic.” Emergency Medicine Clinics of North America, vol. 28, no. 3, 2010, pp. 611-631.
Inouye, Sharon K, et al. “Delirium in elderly people” The Lancet, vol. 383, no. 9934, 2014, pp. 911-922.
Inouye, Sharon K. “Clarifying confusion: The Confusion Assessment Method.” Annals of Internal Medicine, vol. 113, no. 12, 1990, pp. 941-948.
Inouye SK. The Confusion Assessment Method (CAM):Training Manual and Coding Guide. 2003; New Haven: Yale University School of Medicine.
Lewis LM et al. “Unrecognized delirium in ED geriatric patients.” American Journal of Emergency Medicine. vol. 13, 1995, pp. 142-45.
Monette, Johanne, et al. “Evaluation of the confusion assessment method (CAM) as a screening tool for delirium in the emergency room.” General Hospital Psychiatry, vol. 23, no. 1 , 2001, pp. 20-25.
Rosen, Toney, et al. “Assessment and Management of Delirium in Older Adults in the Emergency Department.” Advance Emergency Nursing Journal, vol. 37, no. 3, 2015
Management of Myasthenia Crisis in the ED
Written by: Kumar Ghandi, MD (NUEM PGY-3) Edited by: Arthur Moore, MD (NUEM Alum ‘18) Expert commentary by: Luke Rosiere, MD (NUEM Alum ‘12)
What do I need to know about myasthenia gravis?
Myasthenia gravis is the most common disorder of neuromuscular transmission. The disease can manifest as a combination of weakness in ocular, bulbar, and most importantly respiratory muscles. Myasthenia gravis is disease of the neuromuscular junction, in which autoantibodies are directed against nicotinic acetylcholine receptors (AChR) located on the postsynaptic end plate. These autoantibodies not only induce complement mediated destruction of receptors, but compete with acetylcholine for binding at the remaining receptors [1].
In over 50% of cases myasthenic patients present with ocular symptoms including blurred vision, ptosis, diplopia. 15% of patients present with bulbar symptoms including ptosis, dysarthria, dysphagia, and fatigue when chewing [2]. Isolated respiratory failure is a rare sign. It is important to remember that myasthenic patients will often present specific muscle complaints and not a generalized muscle fatigue [2].
What is myasthenic crisis?
Acute myasthenic Crisis is defined as acquired myasthenia gravis severe enough to require intubation and mechanical ventilation [3]. Typically, between 10-20% of myasthenia gravis patients will experience an episode of myasthenic crisis, most commonly within the first two years [4].
Myasthenic Crisis vs. Cholinergic Crisis
Though cholinergic crisis is a quite rare it is important to differentiate cholinergic crisis from myasthenic crisis in the early evaluation of these patients. Cholinergic crisis is precipitated by excess use of cholinesterase inhibitors. This can manifest as both nicotinic and muscarinic toxicity. Nicotinic symptoms include weakness and fasciculations, while muscarinic symptoms include diaphoresis, tearing, increased oral secretions, diarrhea, and bradycardia [4].
What can trigger myasthenic crisis?
Common triggers for myasthenic crisis include infection, surgical intervention (thymectomy), pregnancy, childbirth, or tapering of immunosuppressive medications [5]. Medications are another large source of triggers for myasthenic crisis [1] [3] [6]. 50% of patients who receive treatment with high dose corticosteroids develop an early exacerbation and 10% will go on to require mechanical ventilation [6].
What are the priorities of management of myasthenic crisis in the ED?
The tried and true emergency medicine axiom of Airway, Airway, Airway is critical for the management of myasthenic patients. Recognition of impending respiratory arrest, early intubation, and placement of a definitive airway has reduced mortality from 40% in the 1960’s to less than 5% today [7]. Evaluation of respiratory function and airway management for patients in myasthenic crisis presents multiple unique challenges in the ED.
How do I best assess respiratory function in the emergency department?
Signs and Symptoms
Patients may describe dyspnea when laying flat. This is thought to be secondary loss of diaphragmatic excursion due to increased dependence of gravity during a myasthenic crisis [4]. Patients may also demonstrate severe dysphagia, with weak cough and inability to clear secretions. Other signs respiratory weakness includes, pausing mid-sentence to take a breath, use of accessory muscles, and tachypnea with shallow breathing [3]. A simple bedside evaluation includes having a patient take a deep breath and count out loud to twenty-five. If they are unable to reach five without pausing for a breath the patient may be bordering on respiratory failure [7].
Forced Vital Capacity
Assesses function of both inspiratory and expiratory muscle function.
Assess in both supine and sitting position as respiratory function tends to decline when supine.
Elective intubation should be considered for FVC <15 [1].
Maximal Inspiratory Pressure/Negative Inspiratory Force
Patient maximally inhales against a closed valve and force at the mouth is recorded.
Inspiration is a negative generating force, thus the more negative the value the stronger the inspiratory effort.
NIF of 0 to -30 cm H20 indicates profound respiratory muscle weakness.
Elective intubation should be considered for those with a NIF in the range of 0 to -30 [3].
Use of these tools are best utilized both in combination and serially with repeat assessment every two hours to assess for progressive respiratory failure. This data allows for objective assessment of respiratory function and assessment for the necessity of a well-controlled elective intubation.
What do I need to consider before intubating?
Neuromuscular Blockers in Myasthenia Gravis:
Due to the pathophysiology of the disease, myasthenic patients due to mechanism of disease are typically resistant to neuromuscular blockade with depolarizing neuromuscular blocking agents such as succinylcholine. Due to the decreased number of receptors myasthenic patients typically require 2.6 times the dose [8]. In addition, use of cholinesterase inhibitors typically used to treat myasthenia gravis can prolong the effect of succinylcholine.
Though succinylcholine is still considered a safe agent, higher doses and inhibition of metabolism can lead to an unpredictable and lengthy paralysis. Typically, a non-depolarizing agent such as rocuronium is the preferred paralytic drug of choice. Myasthenic patients can be sensitive to non-depolarizing agents, starting at 0.1 to 0.2 mg/kg can often provide sufficient neuromuscular blockade [8].
Ventilator Settings:
Initial setting on the ventilator include:
AC/VC mode
Tidal volumes of 8-10 cc/kg of ideal body weight
PEEP 8-15cm H20 to prevent atelectasis and minimize work of breathing [4].
Can I use NIPPV to prevent intubation in myasthenic crisis?
Noninvasive ventilation has previously been shown to prevent intubation in patients in myasthenic crisis. Up to 20% of patients with myasthenic crisis could potentially be managed with non-invasive ventilation. Given the propensity for difficulty swallowing, these patients must be able to protect their airway and manage their secretions in order for NPPV to be a safe alternative. Early NIV has been shown to be associated with reduction in days of ventilator support, length of stay in the ICU, and rate of reintubation [9].
Disposition and Post Emergency Department Care
It is critical to alert Neurology early in the course of a possible myasthenic crisis. Often, myasthenic patients are well known to the department of Neurology, as these patients often frequent the hospital for mild exacerbations of myasthenic symptoms. Myasthenic crisis patients often require close observation in an ICU given their high risk for respiratory failure or need for ventilator management. Treatment options once leaving the ED often include, plasma exchange (PLEX) or intravenous immunoglobulin (IVIG). The table below serves as an excellent comparison for PLEX vs IVIG, knowing the modality of choice from the Emergency Department can help determine the vascular access needed to perform each modality [4].
Expert Commentary
Myasthenic crisis is such a great topic. I can't think of another case that employs such a breadth of expertise.
While it is most exciting to talk about how to manage a patient in crisis, it can't be understated how critical we are in preventing such a crisis. Look closely at that list of precipitating medications. At least half of those are on my Top 20 most common prescription list. Myasthenia is like prolonged QT. If you don't think twice about your therapies, you can put this patient in a heap of trouble and plant them in the ICU for 2 weeks. So, first thing I can say is, if you ever see myasthenia gravis on a PMH, whether they're there for a UTI or a hang-nail, think twice and three times about every medication you write for (including IV contrast).
When it comes to the recognition and management of a crisis, the above description is great.
As you would suspect by the word "crisis," this won't be subtle.
I'll add the following bullets:
1) Great patient to monitor with continous capnography. It will be your quickest indicator of deteriorating ventilation.
2) Keep myasthenia in your differential for any undifferentiated hypercarbic respiratory failure. Before you paralyze and intubate, try and get a brief history and physical to assess for intermittent weakness, bulbar nerve palsies, etc. Otherwise, this potential diagnosis may be missed for days until they can get this patient off the vent
3) Non-invasive ventilation sounds great, but probably won't help that much. These crises last for days, and you can't keep a mask on for that long. These patients often have dysphagia, so will aspirate. Many patients have excess secretions that are better managed through an endotracheal tube. If they need positive pressure, you're best off securing the airway before it's full of vomit.
4) Succinylcholine 2 mg/kg or rocuronium 0.6 mg/kg. Anesthesia literature often recommends puny doses (if any at all) of NMBAs. They're expecting to extubate that patient in 3 hours and fear prolonged paralysis. We are expecting them to be on the vent for days. You need to ensure you have adequate intubating conditions, and paralytics will help. Don't forget to keep your patient well-sedated if you think they're still paralyzed.
5) We're not expected to decide how to cure this crisis. Call neurology and they'll tell you their preference. You'll probably start steroids and they'll decide if they want IVIg or plasma exchange. None are going to work in 5 minutes anyway, so they can deliberate a little.
Luke Rosiere, MD
Northwestern University Emergency Medicine Class of 2012; Attending Physician at Northwestern Medicine Central DuPage Hospital
How To Cite This Post
[Peer-Reviewed, Web Publication] Ghandi K, Moore A (2018, November 5). Management of Myasthenia Crisis in the ED. [NUEM Blog. Expert Commentary by Rosiere L]. Retrieved from http://www.nuemblog.com/blog/myasthenia
Other Posts You May Enjoy
Resources
Adams, James, and Erik D. Barton. Emergency medicine: clinical essentials. Philadelphia: Elsevier Saunders, 2013. Print.
Clinical Manifestations of Myasthenia Gravis . (n.d.). Retrieved April 19, 2017, from https://www.uptodate.com/contents/clinical-manifestations-of-myasthenia-gravis
Myasthenic Crisis . (n.d.). Retrieved April 21, 2017, from http://www.uptodate.com/contents/myasthenic-crisis
Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist 2011; 1:16.
Berrouschot J, Baumann I, Kalischewski P, et al. Therapy of myasthenic crisis. Crit Care Med 1997; 25:1228.
EM:RAP. (2015, September). Retrieved April 20, 2017, from https://www.emrap.org/episode/september/drugsnottousein
EM:RAP. (2006, January). Retrieved April 21, 2017, from https://www.emrap.org/episode/january2006/myasthenia
Anesthesia for the patient with myasthenia gravis. (n.d.). Retrieved April 19, 2017, from http://www.uptodate.com/contents/anesthesia-for-the-patient-with-myasthenia-gravis
Seneviratne J, Mandrekar J, Wijdicks EF, Rabinstein AA. Noninvasive ventilation in myasthenic crisis. Arch Neurol. 2008;65:54–58
The PATCH Trial
Written by: Andrew Berg, MD (NUEM PGY-3) Edited by: Ryan Huebinger, MD, (NUEM Grad 2017) Expert commentary by: Stephen Trevick, MD
Intro:
Hemorrhagic strokes, while accounting for less than 20% of incident strokes, contribute to half of all stroke-related deaths and long-term disability, totaling up to 47 million life-years lost [1]. Unlike ischemic strokes which have the potential to be intervened upon, non-surgical treatment of hemorrhagic stroke is limited. Hemorrhagic stroke associated with the use of non-reversible antiplatelet agents can be problematic, and the goal should be to try to limit the extent of the hemorrhage. In this day and age of increasing myocardial ischemia, percutaneous coronary intervention, and ischemic stroke leading to increasing antiplatelet usage, the attempted reversal of antiplatelet agents with platelet transfusion seems like a logical step. However, it is unclear if this is efficacious (both in the short and long-term) or if there are potential harms as studies are limited. The PATCH Trial looked at the use of platelet transfusion after acute spontaneous intracerebral hemorrhage in people taking antiplatelet therapy to determine if there was any impact on long-term functional outcomes.
The Study:
Baharoglu, M. Irem, et al. "Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial." The Lancet 387.10038 (2016): 2605-2613.
Study Design:
This was a 6-year multicenter (60 hospitals in Europe), parallel-group trial that randomized 190 patients with non-traumatic supratentorial intracerebral hemorrhage while on antiplatelet therapy to standard care or standard care plus platelet transfusion and performed both an intention-to-treat and as-treated analysis of the outcomes.
Population:
Inclusion criteria:
- 18 years or older with non-traumatic supratentorial intracerebral hemorrhage confirmed by brain imaging
- Glasgow Coma Scale ≥ 8
- Platelet transfusion could potentially be initiated within 6 hours of symptom onset and within 90 minutes of brain imaging
- On antiplatelet therapy with either a COX inhibitor (aspirin or carbasalate), ADP receptor inhibitor (clopidogrel) or an adenosine-reuptake inhibitor (dipyridamole) for at least 7 days prior to the ICH.
- Pre-ICH mRS (modified Rankin Score) score of 0 or 1 only, suggesting no prior disability.
Exclusion criteria:
- Imaging findings suggestive of epidural or subdural hematoma or those needing surgery within the next 24 hrs of admission
- Imaging suggesting underlying aneurysm or AVM
- Prior adverse reaction to platelets
- Use of a vitamin K antagonist
- Known coagulopathy
- Imminent death
Patient selection flow chart:
Intervention protocol:
The patients were randomized to either standard care or standard care plus platelet transfusion. Both groups had a repeat brain imaging 24 hours after intervention.
Standard care was not defined in the protocol, but was assumed to be given according to contemporary European and national guidelines.
Platelet transfusions were initiated within 6hrs of intracerebral hemorrhage symptom onset and within 90 min of diagnostic brain imaging. Those patients on clopidogrel received 2 units of platelets, all others received one unit as determined by in-vitro experiments.
Outcome Measures
The primary endpoint was difference in functional outcome at 3 months after randomization scored with the mRS, as was done for the inclusion criteria, which was scored by a physician or nurse not involved with the medical treatment. Secondary clinical endpoints further stratified these outcomes into survival, poor outcome defined as mRS of 4-6 and poor outcome defined as mRS of 3-6. Another secondary outcome was median absolute intracerebral hemorrhage growth in mL after 24 hours on brain imaging. Safety outcomes and other serious adverse events were also recorded.
Results
For the primary endpoint of functional outcome difference at 3 months, there was an increase in odds toward death or dependence at 3 months in those that received platelet transfusions, both adjusted and unadjusted (adjusted common OR 2.05, CI 1.18-3.56, p=0.0114). Secondary analysis and serious adverse events are listed in the following graphs:
Interpretation
For the primary outcome, there was increased odds of a poorer functional outcome after 3 months among those who received platelet transfusions compared to those who just received standard care. In the secondary analysis (which stratifies these functional outcomes further into smaller categories), showed there was a significant poorer outcome at 3 months within the mRS 4-6 category (higher disability) for those that received platelets. All the other subcategories were insignificant. Median ICH growth did not differ significantly between the two groups at 24 hours. Among the serious adverse events, the only minimally significant difference was an increased odds of an adverse event due to ICH as a whole in the platelet transfusion group, though this may be explained by differences in the baseline characteristics of the different arms of the study.
Strengths
- While there was some crossover between the two study arms, there was an intention-to-treat analysis as well as an as-treated analysis.
- Follow-up was strong without attrition.
- The physician/nurse that performed the mRS after 3 months was blinded to randomization.
- Their intended inclusion/exclusion criteria were strong.
Weaknesses
- Looking at the baseline characteristics, there were several patients included in the trial that should have been excluded by criteria (GCS <8, infratentorial ICH location, etc).
- Even though the paper states that the baseline patient characteristics were balanced between the two arms, it appeared that as a whole, the patients who were randomized to receive platelets were sicker (lower GCS, more patients with ICH volume >30mL, both the infratentorial ICH patients).
- The majority of patients were on a COX inhibitor (>90% of patients), with very little representation of the other antiplatelet agents
- This study could have been strengthened with platelet function testing to evaluate for modified treatment effect.
Internal/external validity
External validity could be questioned as this study presumably included only European patients (although race was not specifically mentioned). This study also likely cannot be generalized to non-COX inhibitors given how few patients were on ADP inhibitors, such as clopidogrel.
Internal validity is questioned with the inclusion of patients who met exclusion criteria and should have been excluded. The authors comment on this being an issue for several emergency-department studies given situational urgency.
Future Directions
Given the high prevalence in use of Plavix or Ticagrelor, there should be a study that includes more ADP inhibitors.
Summary:
- This was a European multi-center randomized trial comparing the functional outcomes of patients with spontaneous intracerebral hemorrhage on antiplatelet therapy, when they received either standard care or standard care plus platelet transfusion.
- The study included 190 patients from either Netherlands, UK or France.
- Their results indicated that platelet transfusion did not benefit patients from a functional outcome after 3 months, and in fact, may be associated with worse outcomes. Although there were several weaknesses in the study’s execution, these results seem to be significant enough to have some validity.
- There was no difference in reported/observed immediate (<24 hour) outcomes on imaging between the two groups
Expert Commentary:
ICH is a devastating disease, and often one which we often must watch powerlessly, despite the acuity of presentation. One of the first goals in ICH management is to prevent further bleeding. Platelets for aspirin reversal seemed promising, since it has been documented that patients on aspirin have more hematoma expansion and worse outcomes, as well as clear anecdotal evidence from surgeons that platelet infusion in aspirin users makes an overt difference intra-op. So why should PATCH have been negative?
Given the complexity of factors leading to hematoma expansion and subsequent hospitalization, it is hard to drive outcomes with any one intervention. However, even the rate of hematoma expansion was unchanged. Even though the irreversible binding of Aspirin usually takes many days to wash out, serum half-life is about 15-20 minutes (with active metabolites lingering a few hours). One thing to remember is that transfused platelets themselves can have time-limited efficacy due to immune-related consumption and inactivation. While even a temporarily effective transfusion can help stop brief bleeding such as in the OR or during acute stabilization, it is unlikely to prevent the stuttering hematomal expansion of ICH.
Platelets, unlike other blood products, must be stored at room temperature. Therefore, platelet transfusions are associated with high risks of transfusion reactions. It is theorized that some platelets may become activated prior to transfusion and can therefore also be associated with a risk of pathological clotting. These factors could blunt any potential benefit of treatment.
Intracerebral hemorrhage is a rarer and more heterogenous illness than stroke or MI. This trial is as high of quality as we are likely to obtain on the topic. When applying the results of the PATCH trial, it is important to remember that no patients with platelet counts below 100k were enrolled, so transfusions to meet that goal may still be performed. Also, it is still acceptable to transfuse for procedures or surgery. The study did not explore any sort of functional assays, namely Platelet Function Assay (PFA) or Platelet Aspirin Assay (PAA).
Stephen Trevick, MD
Neurocritical Care Fellow, NUEM
How to cite this post
[Peer-Reviewed, Web Publication] Berg A, Huebinger A (2018, May 14 ). The PATCH Trial. [NUEM Blog. Expert Commentary by Trevick, S]. Retrieved from http://www.nuemblog.com/blog/PATCH
Posts you may also enjoy
Resources
1. Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013:the GBD 2013 Study. Neuroepidemiology 2015; 45: 161–76.
The Migraine Cocktail: Emergency Department Management of Headaches
Written by: Vidya Eswaran, MD (NUEM PGY-2) Edited by: Danielle Miller, MD, (NUEM PGY-3) Expert commentary by: Seth Trueger, MD, MPH
Expert Commentary
Thanks for this great overview over ED headache management. Our approach to headaches has matured in recent years, largely because of a bunch of great studies by Ben Friedman’s group at Montefiore (COI: his brother and I were residency classmates); see Headache guidelines (he’s the first et al in the Orr paper cited above, REF); his Annals Expert Clinical Management paper [REF]; and his FOAM post at ALiEM.
My general approach to headaches:
First: Is there a dangerous cause?
Is the headache similar to their prior headaches in character, location, magnitude, timing, associated symptoms? Are there concerning features (exertional, vomiting, personal or family history of aneurysms)? Was it maximal at onset (or sudden/severe)? I find it helpful to ask: “what were you doing when it started?”; “how bad was it when it started?”; “when was it the worst?”; and only after listening for a while, I backdoor into whether it is typical for them (e.g. “it’s usually on that side and you’re nauseated when you get a headache like this…?”). I’ve gotten myself in trouble by asking up front if it’s the same as usual or “worst headache of your life” – even if they don’t mean to, patients sometimes seem like they are trying to validate why they came to the ED (to us or to themselves). Of course, none of these questions are black and white and there’s a lot of room for clinical judgment; one minor deviation from typical headache does not mandate imaging. Patients come to see us for our expertise and often find it reassuring that we’ve listened and examined them and aren’t concerned.
Second: Symptom management
Turn off the lights
Even migraines without frank photophobia often feel better in a darker room. I usually turn off the lights as soon as I walk in. No reason to wait.
Metoclopramide
Any of the dopaminergic antiemetics are effective; I generally use whichever is typically used in my ED (and doesn’t have to come from pharmacy). IM works fine if the patient doesn’t have an IV – most headache patients don’t need labs or IV fluids so no reason to start one routinely and a lot of even severe headaches are suitable for fast track. But they just don’t seem to work PO.
APAP/NSAIDs
I make sure the patient is up to their appropriate daily dosing of acetaminophen or ibuprofen; with appropriate consideration of contraindications, they might make a difference so why not? PO ibuprofen is likely as effective as ketorolac, and getting a shot doesn’t seem to have a placebo effect (if nothing else, this study is worth reading for the amazing design [REF]; summarized in an accompanying editorial on placebos [REF]).
Steroids
Steroids don’t fix the headache today but they decrease recurrence in some patients. I don’t give them to everyone, but for patients who get headaches in groups, have been having headaches for a while, or are just miserable enough, I give 10mg of dexamethasone.
No diphenhydramine
Diphenhydramine doesn’t work for headaches [208 patient RCT, REF].
Diphenhydramine doesn’t prevent metoclopramide-associated akathisia [REF; REF] (which in my experience, is much less common than the literature describes). Midazolam has some effect for prophylaxis [REF], but the rate of akathisia is low enough that I don’t think it’s worth the risks (or extending LOS due to zonking out the patient). [n.b. the same for avoiding prophylaxis for ketamine sedations; REF] If the patient gets akathisia [or an emergence reaction], then I give midaz.
I’ve heard people suggest diphenhydramine works by knocking out the patient and as any migraine sufferer knows, the best treatment is probably sleep, but the evidence suggests that diphenhydramine just doesn’t add much.
That being said, I pick my battles and I don’t knock it our of nurses hands or reprimand the residents every time or even fight with patients if they really think it helps. But never push IV Benadryl – it gets you high [REF].
No fluid
Unless the patient has been vomiting a lot or has another reason to be volume down, there is little reason to give IV fluids [REF], and in my experience, it locks the patient’s LOS into at least however long the bag takes to drip in, and of course patients who need fluids the least are most likely to have the most positional IVs….
Triptans
I’ll be honest, I don’t give triptans, except in the rare cases where patients know they work for them. Usually the patient’s already taken their home dose, and it seems like most patients have missed the window for effectiveness by the time they’re seeing me. I admit that I’m behind the science and this probably has more to do with practice patterns during my training. I’m open to being convinced.
Sleep
If the patient falls asleep and my ED has the bandwidth, I rarely wake them up. Seems worth giving up the room for a few hours for what’s essentially curative therapy for a miserable condition.
Occipital Nerve Block
If the headache is occipital and radiates forward at all, it could be occipital neuralgia, which often responds quite well to occipital nerve blocks. The diagnosis is not very scientific, but the potential treatment is very simple and, if nothing else, just takes a few minutes of my time and a little pain for the patient, which might provide them hours of relief. I recommen a bupivicaine occipital nerve block to any patient who I think could have occipital neuralgia, review the anatomy, make sure I'm not in the artery, and put in a nice dose of bupivicaine.
No opioids
Opioids don’t work for headache. I never* give opioids for headache. Don’t give opioids for headache.
That being said, the evidence base is surprisingly weak (see REF). My personal experience resonates here; I’ve had a handful of classic migraines, and some were when I was studying abroad in Australia where codeine is OTC. It made me sleepy-ish but didn’t help the headache at all, for whatever that’s worth.
*Rarely, if I see a patient with a pain contract that includes opioids for headaches from a reputable source, I don’t die on that hill.
2nd round: magnesium + (metoclopramide or haloperidol)
If the patient need a second round (or if their headache was terrible to begin with) I throw some IV mag at them. The evidence is weak at best, but it might help and is pretty safe, so why not.
I usually re-dose metoclopramide at this point, but if their current or prior headaches are generally refractory, I often switch to haloperidol (2.5mg IV or IM).
Dispo
Key points in communicating with the patient and evaluating them for discharge:
I don’t have a silver bullet to fix their headache. My goal is to make sure we’re not worried that there is something dangerous going on (“good news! we’re not”) – it’s safe to go home and we have a good, safe plan for follow up (we’re not just kicking them out). While I can’t make the headache go away completely, “my other goal is to get you to the point where you can be miserable here or miserable at home” and we can safely discharge with return instructions and follow-up; I consider Neuro or headache specialist referral if it seems appropriate.
I really think that articulating a lot of these steps is helpful. Much of what we do implicitly is not clear to the patient – our across-the-room gestalt, our assessment and thought processes. Patients want to be listened to, they want to know what to expect, they want to know what to do, and they want a doctor who cares.
Seth Trueger, MD, MPH
Assistant Professor, Northwestern Emergency Medicine
How to cite this post
[Peer-Reviewed, Web Publication] Eswaran V, Miller D (2018, April 30 ). The Migraine Cocktail: Emergency Department Management of Headaches. [NUEM Blog. Expert Commentary by Trueger, S ]. Retrieved from http://www.nuemblog.com/blog/headache
Posts you may also enjoy
The ED Guide to Neuroimaging: Part 1
Written by: Justin Seltzer, MD (NUEM PGY-1) Edited by: Andrew Cunningham, MD, (NUEM PGY-3) Expert commentary by: David Rusinak, MD
Neuroimaging, mainly using CT, has become an indispensable part of our emergency diagnostic process, but, all too often we rely on radiologists to interpret what we ordered. The goal of this multi-part blog is as follows:
To cover the basics of how to look at a CT brain and quickly identify life threat
Review the literature supporting the major ED indications
Discuss special considerations, such as when to use contrast, angiography, or MRI instead.
Systematic Reading of a CT Brain
The first portion of this blog will focus on how to read a CT brain quickly with a focus on life threats.
The classic mnemonic, “Blood Can Be Very Bad,” is a pathology oriented, step-wise method to look for blood, cistern changes, and alterations to the brain parenchyma, ventricle appearance, and bony anatomy. Applying this approach to each image cut individually can help reveal subtle findings that would otherwise be easily missed by quick scrolling.
Blood:
Blood can collect both intra-axially (parenchymal) and extra-axially (outside the parenchyma).
Figure 1
Classically, spontaneous intra-axial bleeding originates in deep structures such as the basal ganglia and thalamus (Figure 1).
In the setting of trauma, intra-axial bleeding is often ipsilateral or directly contralateral to the injury site (coup-contrecoup) but can be anywhere
Inferior frontal and anterior temporal lobes are high risk for traumatic contusions due to close proximity to bone (Figure 2)
Figure 2
Extra-axial bleeding is defined by location: mainly subdural, epidural, subarachnoid, and intraventricular hemorrhages. The patterns for these are well known and readily identified, however below are some key points on extra-axial bleeding.
Finding chronic subdural hematomas can be difficult as older blood and grey matter are similar appearing
Mass effect, abnormal appearing brain folds on that side, and use of coronal reconstructions can help identify
Subarachnoid hemorrhage becomes difficult to see within hours to days but acutely is often observed well in the cisterns (see below)
Be careful not to mistake choroid or pineal calcifications for hemorrhage
Don’t forget about scalp hematomas
Cisterns:
The cisterns are not ventricles but rather outpouchings of the subarachnoid space. When evaluating a CT brain the following, certain cisterns have clinical relevance for potential herniation syndromes, layering of subarachnoid blood, and/or the significant structures that run through them. Figures 3-5 show the locations of the major cisterns described below.
Figure 3
Figure 4
Suprasellar: Located in the area of the sella turcica, forms a pentagon/star shape
Classic location of subarachnoid hemorrhage due to proximity to circle of Willis
Obliteration associated with downward transtentorial (i.e. uncal) herniation or due to severe elevated ICP
Perimesencephalic cistern: A group of interconnected basal cisterns surrounding the midbrain (mesencephalon), important location of subarachnoid hemorrhage, may see effacement (reduction or loss) with tonsillar herniation
Interpeduncular: Located in the area of the cerebral peduncles
Quadrigeminal: Classically forms a W shape, obliteration associated with upward herniation
Ambient and crural: Connections between quadrigeminal and interpeduncular cisterns
Cerebellopontine: Located between anterior cerebellum and lateral pons, synonymous with area of cerebellopontine angle
Cisterna magna: Located between the cerebellum and medulla, receives fourth ventricular CSF outflow (Figure 4)
Prepontine: Located at the anterior aspect of the pons
Figure 5
Brain parenchyma:
CT allows for gross evaluation of the major structures as well as a differentiation of grey and white matter by Hounsfield units. The focus here is major parenchymal disruptions.
Mass lesions, mass effect, midline shift: Because of the fixed nature of the skull, mass lesions of any type easily exert pressure on the surrounding tissue (mass effect) that can result in increased ICP, midline shift, and herniation.
Midline shift is measured in millimeters of displacement of the septum pellucidum at the level of the foramen of Monro from the midline of the skull
Ischemic changes: depending on the size of the involved territory and duration, may be subtle or obvious density or architectural changes.
Early signs of infarction: reduced grey-white matter distinction and loss of insular hyperdensity
Ventricles:
The ventricular system is where CSF is produced and the route by which it travels into the subarachnoid space. The lateral ventricles drain via the foramina of Monro to the third ventricle, which then drains via the cerebral aqueduct (aqueduct of Sylvius) to the fourth ventricle and then to the cisterna magna and the rest of the subarachnoid space via the median and lateral apertures (foramina of Magendie and Lushka, respectively). Figures 3-5 also show the locations of the major ventricles.
Interruption of ventricular CSF flow will cause proximal ventricular dilation that helps localize the level of obstruction
If unsure between hydrocephalus and atrophy, dilation of temporal horns of the lateral ventricles can be helpful as it occurs in hydrocephalus involving the lateral ventricles but not with hydrocephalus ex vacuo
Bones:
Intimate knowledge of bony anatomy is not essential fracture evaluation. However, it is crucial that the bony anatomy be viewed with a dedicated bone window. Skull and facial fractures can be subtle and the presence of blood, especially an epidural hematoma, may help localize them. As noted above, soft tissue findings such as scalp hematomas are important to rule out as well.
Key Learning Points and Conclusions
A systematic approach is essential to avoid missing significant findings, especially with complex neuroimaging—remember “Blood Can Be Very Bad”
Immediately look for: blood anywhere (don’t forget the scalp!), effacement of major cisterns, mass effect/midline shift, enlarged ventricles (temporal horns), skull fractures
Older blood, such as a chronic subdural hematoma, can be hard to find and may require different cuts or inference from mass effect or effaced sulci
Signs of infarction may be subtle (more on this later)
In the next installation, we will discuss the major indications for CT brain and the utility of CT for these indications.
Expert Commentary
Overall, this is a very nice approach to head CT interpretation. The classic mnemonic, “Blood Can Be Very Bad,” is not something I’ve heard of before, but it works. Let’s take each search item in turn.
Blood
A helpful way to think about intracranial hemorrhage is to consider the causes of hemorrhage and the most common location for each pathology. Common causes include trauma, stroke (hypertensive or hemorrhagic conversion of a venous or arterial infarct), neoplasm (primary or secondary), vascular (aneurysm, AVM, dural AV fistula), and spontaneous (anticoagulation, amyloid angiopathy, vasculitis). If you consider the location of each of these pathologies, the hemorrhage will typically be primarily in this location. A tumor, for example, will cause a parenchymal bleed, a ruptured aneurysm will cause subarachnoid hemorrhage, an AVM will result in a parenchymal bleed, etc. Often with parenchymal bleeds additional imaging, vascular and MRI, as well as follow up imaging will be necessary to determine the underlying cause.
A correction is that subacute hemorrhage, not chronic, has a density similar to gray matter. Chronic subdural hemorrhages are usually very hypodense and easy to detect on CT. So, from a practical perspective, a patient experiencing headaches from subarachnoid hemorrhage that is greater than 3 or 4 days old may be occult by CT. This underscores the role of lumbar puncture and vascular imaging in working up patients with headaches.
Another important concept to keep in mind is window and level when interpreting CTs. Different substances (air, metal, bone, blood, fat, etc) have different and defined densities. The pathologies associated with each of these substances (fractures, edema in the setting of stroke, etc) can be better seen by adjusting the window and level settings. This can be done manually or, typically, PACS viewers have preset brain, bone, lung and soft tissue windows that can be displayed by pressing different numbers on the keypad. Subtle subdural hemorrhages are often only seen with the appropriate window and level that allows distinction of the hemorrhage from the overlying calvarium.
Cisterns
The blood vessels course through the cisterns, so these must be scrutinized for the presence of hemorrhage secondary to a ruptured aneurysm in a patient presenting with an atraumatic headache. The cisterns are also effaced in the setting of mass effect. Mass effect may be from a space occupying lesion; such as a tumor, abscess, or hemorrhage; or from diffuse cerebral edema with generalized brain swelling. Often the absence of something (i.e. patent basal cisterns) can be harder to detect than the presence of something, like hemorrhage. It is, therefore, important to examine the basal cisterns on each case to get comfortable with their normal variation of appearance so that their absence, such as in diffuse cerebral edema, is not missed.
Brain parenchyma
Subtle changes in parenchymal density can be difficult to detect. It is important to get acquainted with ideal window and level settings to uncover subtle parenchymal changes. Also comparison with prior imaging, if available, is necessary to determine the chronicity of parenchymal findings. Understanding where a physical exam finding localizes intracranially can also be very useful- aphasia or left upper extremity weakness localize to very different locations, for example. Lastly, always look at the vessels in the subarachnoid space to identify hyperdense thrombus in the setting of a suspected stroke.
Ventricles
Distinguishing volume loss from ventricular dilatation takes experience to understand the variation of normal across the entire age spectrum. If hydrocephalus is suspected, determining if it is obstructive or communicating can help to understand the underlying cause. The temporal horns are the most elastic portion of the ventricles and dilate first in the setting of hydrocephalus.
Bones
Depressed skull fractures and easy to see on routine bone windows. Things get complicated when subtle non-displaced fractures mimic normal sutures or if the fracture involves the skull base/temporal bones. It is probably not within the normal ED physician’s scope of practice to have a detailed knowledge of skull base anatomy, but if a skull base fracture is suspected (loss of hearing, hemorrhage in the external auditory canal, facial nerve paralysis, etc) it is important or order the proper test for further evaluation, like a temporal bone CT. A helpful tip is to look for subtle foci of intracranial air and soft tissue swelling which may direct you to a subtle fracture.
David Rusinak, MD
Assistant Professor of Radiology, Northwestern Medicine
How to cite this post
[Peer-Reviewed, Web Publication] Whipple T, Gappmeier V (2018, April 23 ). Demystifying the Hand Exam. [NUEM Blog. Expert Commentary by Rusinak D ]. Retrieved from http://www.nuemblog.com/blog/neuroimaging
Posts you may also enjoy
References
1. Shaw AS, Prokop M. Computed Tomography. In Grainger & Allison's Diagnostic Radiology, 6th Edition (2016).
2. McKetty MH. The AAPM/RSNA physics tutorial for residents. X-ray attenuation. RadioGraphics, 1998; 18(1):151-163
3. Cadogan M. CT Head Scan. Life in the Fast Lane. Retrieved from https://lifeinthefastlane.com/investigations/ct-head-scan/.
4. Nadgir R, Yousem DM. Approach and Pitfalls in Neuroimaging. In Neuroradiology: The Requisites, 4th Edition (2017).
5. Mehta A, Jones BP. Neurovascular Diseases. In Grainger & Allison's Diagnostic Radiology, 6th Edition (2016). Chapter 62, 1456-1496.
6. Jones J. Subarachnoid Cisterns. Radiopaedia. Retrieved from https://radiopaedia.org/articles/subarachnoid-cisterns.
7. Nadgir R, Yousem DM. Cranial Anatomy. In Neuroradiology: The Requisites, 4th Edition (2017).
8. Skalski M, Dawes L. Cerebral herniation. Radiopaedia. Retrieved from https://radiopaedia.org/articles/cerebral-herniation.
9. Baron EM, Jallo JI. TBI: Pathology, Pathophysiology, Acute Care and Surgical Management, Critical Care Principles, and Outcomes. In Brain Injury Medicine: Principles and Practice, 2nd Edition (2012). Chapter 18: 265-282.
10. Nadgir R, Yousem DM. Head Trauma. In Neuroradiology: The Requisites, 4th Edition (2017).
11. Waxman SG. Ventricles and Coverings of the Brain. In Clinical Neuroanatomy, 28th Edition (2013).
12. Nadgir R, Yousem DM. Neurodegenerative Diseases and Hydrocephalus. In Neuroradiology: The Requisites, 4th Edition (2017).
13. Galliard F, Jones J. Intraventricular haemorrhage. Radiopaedia. Retrieved from https://radiopaedia.org/articles/intraventricular-haemorrhage
Vertigo: A hint on the HiNTs exam
Written by: William LaPlant, MD (NUEM PGY-2) Edited by: Mitali Parmar, MD, (NUEM PGY-4) Expert
review by: Phillip Chang, MD
What is the HiNTs Exam?
The HiNTs Exam is a screening tool for distinguishing a central cause of vertigo from an acute peripheral vestibulopathy (APV), such as vestibular neuritis. The reason for stratifying is obvious (early intervention for central processes, prevention of adverse outcomes), but the degree of difficulty in correctly stratifying a patient is not.
Why is this important?
A normal neurological exam cannot accurately exclude a central process; 10% of patients with a cerebellar infarct, usually in the medial branch of the PICA, will have isolated vertigo without other associated deficit. [1] In the HiNTs exam derivation paper, only 51% of those with a central process causing vertigo had a neurological sign on exam. [2] While risk factors, including age, can be helpful in risk stratification, there is again no definitive cutoff that can eliminate the risk of a central process.
How to perform the HiNTs exam:
Notably, it can only be used if the patient is currently symptomatic, and is meant for continuous, not intermittent, symptoms. This means in conditions such as benign paroxysmal positional vertigo (BPPV) where symptoms are not continuous, HiNTs exam should NOT be performed.
It is comprised of three components: head impulse, nystagmus and skew
Head Impulse:
How to correctly perform the head impulse test with demonstrations of a negative (A) and positive (B) finding [1].
Ask the patient to relax his/her head and maintain his/her gaze on your nose. Gently move the patient’s head to one side, then rapidly move it back to the neutral position. The patient may have a small corrective saccade. The head impulse test is positive (consistent with peripheral vertigo) if there is a significant lag with corrective saccades. If you can see the correction, it is abnormal. Compare this to the contralateral side; a difference in the speed of correction should be noted. Video for Good Technique
The horizontal head impulse test is consistent with peripheral vertigo if it is positive in one direction only. If there is a lag in corrective saccades in both directions, it may be concerning for central vertigo. This test can also be performed in the vertical plane. A lag in corrective saccades in the vertical plane is always suspicious for a central etiology for vertigo.
Pitfalls: The patient must be awake and cooperative. This is essentially an awake “doll’s eye” that requires conscious fixation on an object. Patients who are mentally impaired, unable to fixate, or sedated cannot do this maneuver. Likewise, anxious patients who are unable to relax their neck are unable to do this procedure adequately. False negatives often result from an inexperienced practitioner being too gentle with the head thrust due to fear of causing neck injury.
Contraindications: Any patient that has head trauma, neck trauma, an unstable spine, or neck pain concerning for arterial dissection. This maneuver may extend the injury. In addition, one should avoid this in patients with known severe carotid stenosis as it may embolize unstable plaque. An acceptable alternative is assessing for ocular dysmetria.
Nystagmus:
Note if nystagmus is present in primary gaze (i.e. looking straight ahead) and/or in lateral gaze. What direction is the fast component? If the nystagmus is worse looking in one direction, with the fast component present in that same direction on contralateral gaze, it is unidirectional and reassuring for peripheral vertigo. If, for example, a patient has right-beating (fast direction to the right) nystagmus with rightward gaze and leftward gaze, this is unidirectional right-beating nystagmus.
Bidirectional nystagmus, I.E fast component to the right with rightward gaze and to the left with leftward gaze, is concerning for a central process, as is vertical nystagmus or pure torsional nystagmus.
Skew:
Again have the patient maintain his/her gaze on your nose. Alternate covering each of the patient’s eyes. A positive result will be the deviation of one eye while it is being covered, followed by correction after uncovering it.
Interpretation:
If the HiNTs exam is entirely consistent with peripheral vertigo (positive head impulse test, unidirectional and horizontal nystagmus, negative test of skew), then, according to the derivation paper, it is 100% sensitive and 96% specific for a peripheral cause of vertigo. [2] While this sounds appealing, there are some caveats:
ALL findings must be present for the HiNTs exam to be invoked. If, for example, the patient has horizontal rightward nystagmus with right gaze and no skew, but has no findings on impulse test, you cannot invoke the HiNTs exam to clear the patient
In the derivation study, it was performed by one practitioner who was a neuro-ophthalmologist using specialized equipment to measure skew.
The study has not yet been validated by a large external group, let alone a large external group of emergency medicine providers. We do not know the sensitivity of the test in this population. As such, while useful in stratifying patients and documenting low risk patients, it would be prudent to err on the side of caution with moderate risk patients given the limited evidence of the sensitivity and specificity of the HiNTs exam in the hands of emergency medicine providers.
Should l get imaging, and if so, what?
CT scan has low sensitivity (16%) for acute infarction in the posterior fossa, so unless you are only concerned for bleed (CT non con) or dissection (CTA), evaluating the patient with just a CT brain essentially commits the patient for an MR stroke rule out. [1] Notably, even initial MRI (48 hours from symptom onset) is falsely negative in 12%, so a patient with a concerning story and exam may require repeat imaging. [1]
As such, there are three possible pathways for these patients: no imaging in the lowest risk, CT vs CTA for patients with concern for bleed or dissection, or MRI +/- CT for ischemic stroke rule out.
Bonus Facts
In the HiNTs derivation paper, patients presenting with central vertigo were more likely to have headache or neck pain (38% vs 12%), a neurological sign on exam (51% vs 0%) including truncal instability (the inability to sit upright unassisted [34%]. [2]
Documentation
Here is an example of one way to document the HiNTs exam. As a default, a negative exam is used. Please feel free to use and adapt it.
Head impulse test: loss of fixation with corrective saccades when head turned to the ***
Nystagmus: unidirectional, horizontal ***-beating nystagmus
Skew deviation: grossly absent
Posts You May Also Enjoy
How to cite this post
[Peer-Reviewed, Web Publication] LaPlant W, Parmar M (2018, Jan 15). Vertigo: A hint on the HiNTS exam. [NUEM Blog. Expert Review By Chang P]. Retrieved from http://www.nuemblog.com/blog/hints.
References
1. Nelson J a, Viirre E. The clinical differentiation of cerebellar infarction from common vertigo syndromes. West J Emerg Med. 2009;10(4):273-277.
2. Kattah JC, Talkad A V., Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: Three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40(11):3504-3510. doi:10.1161/STROKEAHA.109.551234.
The ATACH-2 Trial: Blood Pressure Control in ICH
Written by: Jessica Bode, MD (NUEM PGY-3) Edited by: Michael Macias, MD, (NUEM class of 2017) Expert Commentary by: Andrew Naidech, MD, MSPH
Introduction
Hemorrhagic stroke is the second-leading cause of strokes (behind ischemic strokes). The 30-day mortality from intracerebral hemorrhage (ICH) ranges from 35 to 52 percent, with one-half of these deaths occurring within the first two days[1]. A 2010 systematic review estimated that only between 12 and 39 percent of patients go on to achieve independent function[2]. Somewhat intuitively it has long been thought that aggressive blood pressure (BP) control may improve outcomes by limited hemorrhage expansion.
The INTERACT2 study examined the efficacy of intensive systolic blood pressure (SBP) reduction to a goal of <140 mmHg over the course of 1 hour and within a 6 hour window of symptom onset as measured by death and disability. While initially touted as a positive study further examination of the methodology revealed significant differences in the two arms only with ordinal analysis. When comparing this aggressive intervention to the standard treatment SBP goal of <180, the 2013 study found no significant difference between treatment arms. [3]
The 2016 ATACH-2 study sought to reexamine this question: Does aggressively lowering blood pressure lead to decreased death or disability at 90 days?
Study
Qureshi AI et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage (ATACH-2 Trial). NEJM 2016. PMID: 2726234
Study Design
- Randomized, multicenter, two-group, open-label trial (took place in 110 sites in the US, Japan, China, Taiwan, South Korea and Germany)
Population
- Age 18-90
- Intracerebral hemorrhage (< 60 cm3) seen on CT scan with NIHSS score 4 or greater
- GCS ≥5
- Presenting within 4.5 hours of symptom onset (initially within 3 hours but later extended)
- At least one reading of SBP of 180 mmHg or more between symptom onset and the initiation of intravenous antihypertensive treatment
Exclusion Criteria
- Time of symptom onset not reliably established
- Previously known AVM, neoplasm or aneurysm
- Intracerebral hematoma considered to be related to trauma.
- lCH located in infratentorial regions such as pons or cerebellum
- IVH associated with intraparenchymal hemorrhage and blood completely fills one lateral ventricle or more than half of both ventricles
- Subject is considered a candidate for immediate surgical intervention by the neurosurgery service
- Pregnancy or parturition within previous 30 days or active lactation
- Any history of bleeding diathesis or coagulopathy
- Use of warfarin within the last 5 day
- A platelet count less than 50,000/mm3
- Known sensitivity to nicardipine
- Pre-morbid mRS of 4 or greater
- Subject’s living will precludes aggressive ICU management
Ultimately 8532 patients were screened, 1000 were randomized
Intervention Protocol
Patients were assigned to aggressive (SBP goal <140) or standard (SBP goal <180) arms with BP control achieved via nicardipine or, alternatively, via labetalol or diltiazem. Strict control within these parameters was maintained for 24 hours.
Outcome Measures
Primary
- Modified Rankin scale of 4-6 (death or disability) at 3 months after randomization
Secondary
- European Quality of Life–5 Dimensions (EQ-5D) scores at 3 months
- Visual-analogue scale (VAS) at 3 months
- Proportion of patients with 33% or more expansion in volume of hematoma on 24h delta scan
- Safety outcomes (fall in GCS by 2 or increase of NIH Stroke Score by 4)
- Incidence of serious adverse events within 72 hours
Results
“In conclusion, our results do not support the notion that acute reduction to a target systolic blood pressure of 110 to 139 mmHg in patients with intracerebral hemorrhage is more effective in improving functional outcomes than a reduction to a target systolic blood pressure of 140 to 179 mm Hg.”
Aggressive SBP reduction to a target of 110-139 mmHg did not result in lower rate of death or disability compared to the standard treatment goal of 140-179 mmHg. There was no difference between groups in mRS of 4-6 at 3 months (38.7% in intervention arm, 37.7% in control arm).
Interpretation
Strengths
- Multi-centered trial
- Clinically meaningful outcomes
Weaknesses
- During the trial, ATACH-2 expanded enrollment from patients who presented within 3 hours to those who presented within 4.5 hours. As the authors acknowledge in the paper, this could mask a time-dependent loss of benefit but they deem this unlikely as subgroup analyses of INTERACT2 data did not demonstrate this phenomenon.
- Intracerebral hemorrhage grouped as a single entity
- Primary treatment failure (inability to achieve target BP within 2 hours) was seen in 12.2% of patients in the intensive-treatment group vs just 0.8% in the standard-treatment group. It’s difficult to interpret its impact on treatment effect.
- 56.2% of patients were Asian making it difficult to generalize to primarily European populations though when subgroups extracted out again no clinically significant benefit to aggressive therapy was found.
The Bottom Line
As mentioned above, ATACH-2 serves as a rebuttal to the purported positive results of the INTERACT2 trial. On further analysis, the interpretation of INTERACT2 results was somewhat flawed and many experts have ultimately come to view it as a negative trial, consistent with ATACH-2. Dr. Rory Spiegel discusses this in more depth here.
As it stands, decisions should be made in conjunction with neurology and neurosurgery at your institution, but the literature at present does not support aggressive BP control and in fact this practice may lead to harm in the form of decreased cerebral perfusion. Standard SBP target of <180 mmHg should probably be maintained.
Expert Commentary
Intracerebral hemorrhage (ICH) is the most deadly form of stroke and has no FDA approved treatment . This is not to say nothing can be done, and the lack of specific therapies has heightened the importance of optimizing the management. Protocols to improve diagnosis, obtain appropriate imaging, obtain subspecialty care, screen for dysphagia, ensure rehabilitation assessments and accredit Stroke Centers are important. Within these broad strokes, hypotheses about which specific management strategies are likely to improve biomarkers and, subsequently, patient outcomes, can be rigorously tested.
Severe hypertension is both a risk factor and a cause of ICH. Hypertension is plausibly linked to growth of the hematoma, a proximate cause of worse patient outcomes. Thus, it is reasonable to test the hypothesis that lowering blood pressure reduces hematoma growth and, subsequently, improves patient outcomes.
INTERACT2 was negative for its primary endpoint of improved odds of good outcome with a dichotomous ordinal scale, while statistically significant for ordinal regression (a method to determine if there is a shift along the score) and health-related quality of life (HRQoL). ATACH-2 found no evidence of overall benefit to rapid blood pressure lowering, and an increased rate of renal failure. There was no evidence that rapid lowering of blood pressure reduced hematoma growth, which makes the potential mechanism of improved outcomes conjectural.
These studies highlight another problem: Outcomes assessment in clinical trials may be too crude to show meaningful differences between groups. Since INTERACT2 and ATACH-2 were designed, NIH has created, normed, and distributed new benchmarks for HRQoL, the Patient Reported Outcomes Measurement Information System (PROMIS) and Neuro-QOL. We have previously validated PROMIS and Neuro-QOL against ordinal scales, and found they highlight facets of HRQoL that are important, but not as well measured by ordinal scales while being more statistically powerful [4]. They are now generally accepted as pivotal outcomes for clinical research [5].
Aggressive blood pressure lowering is likely to be of some benefit; a target of 140 – 160 mm Hg systolic is reasonable for patients with systolic blood pressure <220 mm Hg pending the next update of evidence based guidelines. Going forward, accurate assessment of patient outcomes with sensitive, powerful scales will make clinical trials more meaningful, and more likely to be positive.
Andrew Naidech, MD, MSPH
Professor of Neurology (Stroke and Neurocritical Care), Anesthesiology, Medical School Sciences, Neurological Surgery, and Preventive Medicine (Health and Biomedical Informatics), Northwestern University
References
- Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology 2006; 66:1182.
- van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010; 9:167.
- Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355.
- Naidech AM, Beaumont JL, Berman M, Francis B, Liotta E, Maas MB, Prabhakaran S, Holl J, Cella D.Dichotomous "Good Outcome" Indicates Mobility More Than Cognitive or Social Quality of Life. Crit Care Med. 2015 Aug;43(8):1654-9. doi: 10.1097/CCM.000000000000108
- Salinas J, Sprinkhuizen SM, Ackerson T, Bernhardt J, Davie C, George MG, Gething S, Kelly AG, Lindsay P, Liu L, Martins SC, Morgan L, Norrving B, Ribbers GM, Silver FL, Smith EE, Williams LS, Schwamm LH.An International Standard Set of Patient-Centered Outcome Measures After Stroke. Stroke. 2016 Jan;47(1):180-6. doi: 10.1161/STROKEAHA.115.010898. Epub 2015 Nov 24.
Cerebral Venous Sinus Thrombosis: The Forgotten Headache
Cerebral venous sinus thrombosis (CVT) is becoming recognized as a more common and treatable disorder in young patients. Unfortunately, headaches are overwhelmingly common complaints in the emergency department (ED), so CVT can be easy to miss since it has confusing clinical and imaging findings. This week we discuss this clinical entity, when to suspect it and what to order.
Modern Headache Management: Best Practices Update
Headache is one of the most common chief complaints for patients in the emergency department (ED). In this week's post we discuss the evidence behind our common management strategies.
Understanding An Enigma: Lumbar Puncture After A Negative CT in SAH
Acute headache is a common emergency department complaint, and in the right clinical setting, subarachnoid hemorrhage can often be high on the differential. We review an article that delves into the data on whether patients with a negative head CT still need an LP. The jury may still be out.
The ED Approach To The Comatose Patient
Approximately 3% of all ED patients arrive in some sort of altered mental state. In this post we dive into the emergency department evaluation of these patients and highlights key components of the physical examination and initial evaluation to help you narrow your differential diagnosis.
A Highly Gifted Juice
An Evidence Based Approach To Opening Pressure in CSF Analysis and its Role in Idiopathic Intracranial Hypertension