Written by: Pete Serina, MD, MPH (PGY-2) Edited by: Laurie Aluce, MD (PGY-3) Expert Commentary by: Timothy Loftus, MD, MBA
Expert Commentary
Kudos to Drs. Aluce and Serina on a well-written, visually appealing infographic on the use and application of d-dimer testing in the ED. I would like to add a couple points of emphasis and elaboration, albeit in a less visually appealing and therefore more cumbersome format…
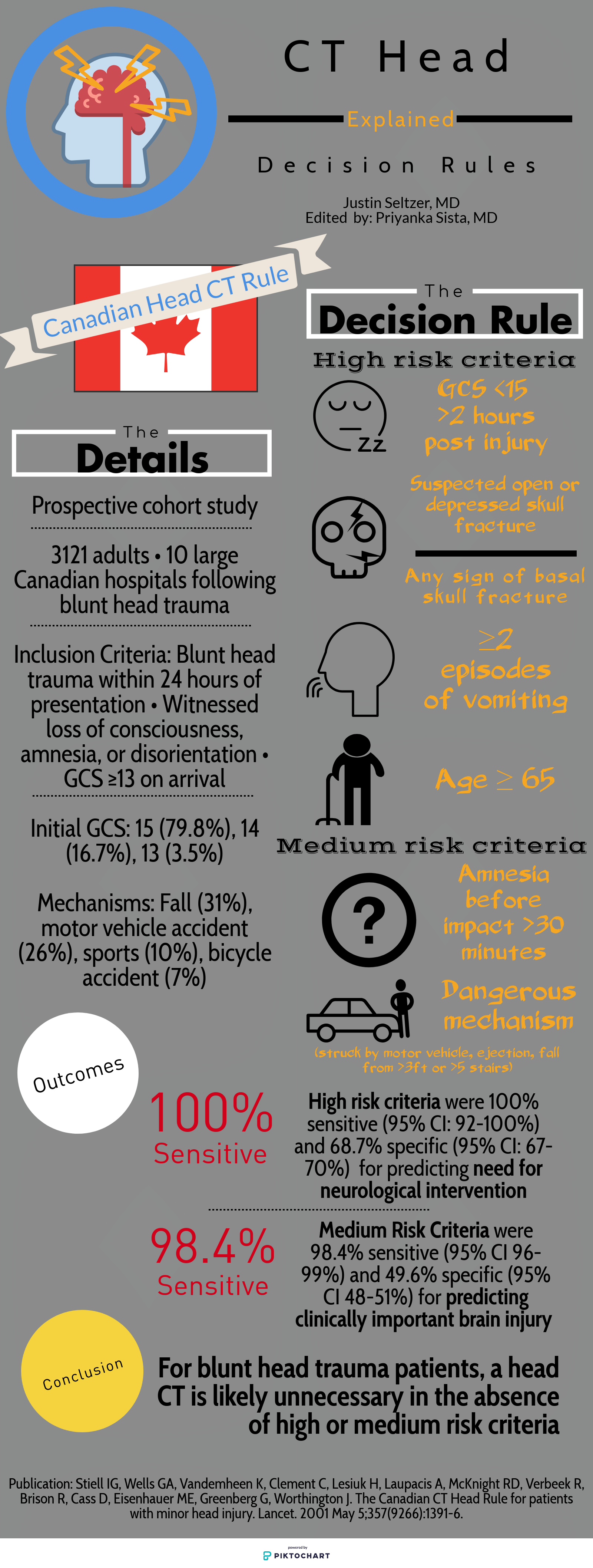
1. The most important step in any diagnostic algorithm for PE is the first question -- do you really think this patient could have a PE? It seems that PE is considered on the differential for nearly every patient in the ED. There’s plenty of data out there to suggest that even seasoned clinicians drastically overestimate the probability of PE.
2. Risk stratification - whether using an experienced physician’s clinical gestalt, Wells, or Revised Geneva Score - is the first step prior to the potential (mis)application of PERC. This can be a common pitfall in the diagnostic evaluation of PE, as PERC is only recommended in the low-risk patient population. There is no evidence to convincingly support its use in non-low-risk populations. Take for example a young cancer patient with dyspnea and pleuritic chest pain - a mistake would be to apply PERC to this patient prior to appropriate risk-stratification.
3. PERC is not perfect - however the evidence is pretty robust. Use caution in settings with a relatively high prevalence of PE. Additionally, PERC is a rule-out criteria, not a risk stratification tool.
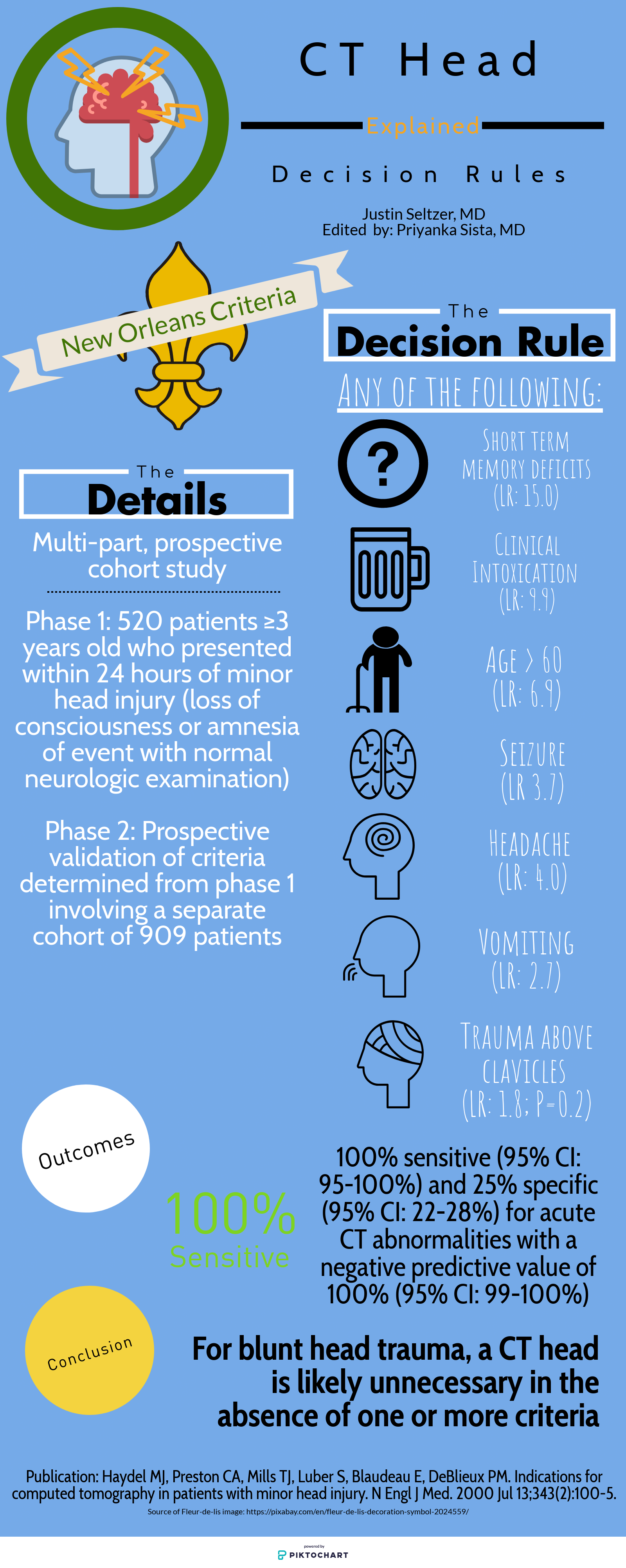
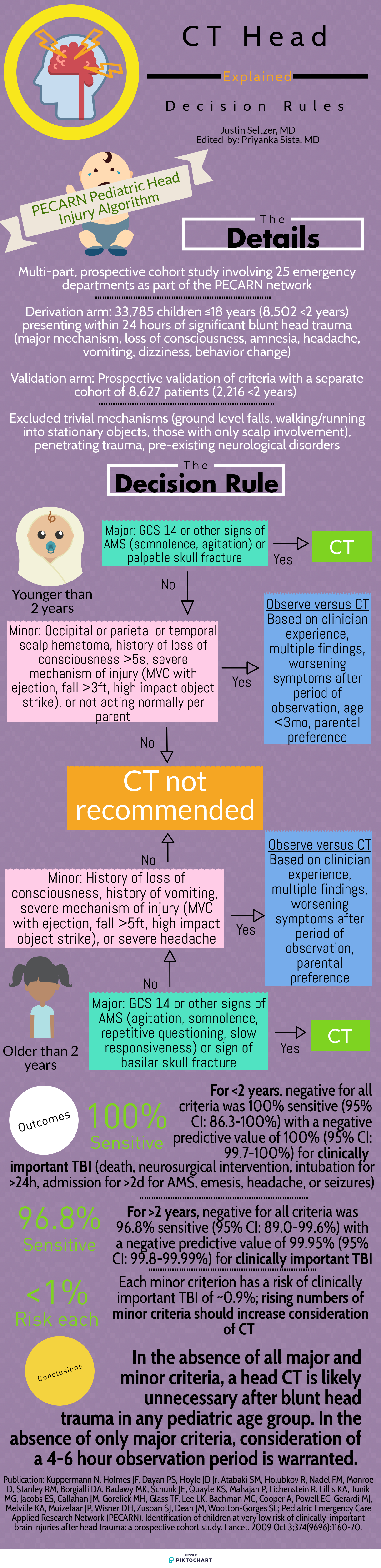
4. While the authors did not mention specifically the use of high sensitivity d-dimer testing in pregnant patients, this is a topic of much discussion as of late. The first study to prospectively evaluate the utility of d-dimer testing in pregnancy was published in 2018 by Righini and co-authors (of Revised Geneva Score fame). Interestingly, the use of d dimer testing in pregnancy is a practice currently recommended against by the American Thoracic Society 2011 guidelines. In the 2018 study, the authors found a clinically meaningful (11%) proportion of patients in whom d-dimer testing could be safely used to exclude PE. As you might imagine, most of this utility was identified in those patients in the first trimester, as d-dimer levels rise during pregnancy (Kline even recommends trimester based cutoffs of 750/1000/1250 although this has yet to be prospectively studied). Further, PE has been cited as the #1 cause of obstetric mortality, which is no laughing matter in the United States where we have many opportunities for improvement with respect to maternal mortality. Muddying the waters further, the YEARS algorithm was also adapted for use during pregnancy. Ultimately, many of us await the next iteration of guidelines to support or optimize our diagnostic decision making for VTE in pregnancy, although the data seem very promising for using d-dimer testing in low to moderate risk patients.
5. I would echo the authors for those in the back - age-adjusting the d-dimer threshold is guideline recommended. Unfortunately, significant variability remains given local practice pattern variation, malpractice environment differences, and differences in assay use.
6. The recent PEGeD study (2019) has furthered the discussion on raising d-dimer thresholds for those with low clinical pretest probability (PTP). Importantly, the authors excluded pregnant patients and those who received “major surgery” within the past 3 weeks from this study. Essentially, this was a study that looked at the application of a higher d-dimer threshold in low PTP patients, also known as a risk-adjusted d-dimer approach. This has the potential to reduce CT imaging by 33% with 0 cases of VTE diagnosed at 3 month follow up.
7. Speaking of reducing CTPA imaging, Dr’s Kline, Courtney, and co-authors have recently published that 2.3% of ED patients undergo CTPA scanning, d-dimer was used in <50% of those patients, and increased d-dimer usage was associated with higher PE yield rate. This finding certainly supports local quality improvement efforts aimed at optimizing the utilization of CTPA within the ED….
Unfortunately, at the end of the day, up to 50% of PEs are diagnosed in patients with no apparent risk factors. That makes everything crystal clear, right?
Great job again by Dr’s Aluce and Serina on a concise, visually appealing, excellent overview of d-dimer testing in for PE in the ED.
References:
Kline J. et al. D-dimer concentrations in normal pregnancy: new diagnostic thresholds are needed. Clin Chem. 2005 May;51(5):825-9. PMID: 15764641
Leung AN et al. An Official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline: Evaluation of Suspected Pulmonary Embolism in Pregnancy. Am J Respir Crit Care Med 2011. Nov 15;184(10):1200-8 PMID: 22086989
Righini, M., et al. Diagnosis of Pulmonary Embolism During Pregnancy. A Multicenter Prospective Management Outcome Study. Ann Intern Med. 2018 Dec 4;169(11):766-773 PMID: 30357273
van der Pol, L. M., et al. Pregnancy-Adapted YEARS Algorithm for Diagnosis of Suspected Pulmonary Embolism. N Engl J Med. 2019 Mar 21;380(12):1139-1149 PMID: 30893534
White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-8.
Timothy Loftus, MD, MBA
Assistant Professor
Department of Emergency Medicine
Northwestern University
How to Cite This Post
[Peer-Reviewed, Web Publication] Serina P, Aluce, L. (2020, April 27). D-Dimer How To. [NUEM Blog. Expert Commentary by Stelter, J]. Retrieved from http://www.nuemblog.com/blog/dimer