Written by: Steve Chukwulebe, MD (NUEM PGY-4) Edited by: Spenser Lang, MD (NUEM ‘17) Expert commentary by: Leah Harris, MD and Kiona Allen, MD
Case
A 38-year-old G8P6 female at 39-weeks gestation presents to your emergency department in active labor. The patient has gotten all her prenatal care at your institution, but has not made it to her due date. She reports her water breaking about 2 hours ago. She describes that the ruptured fluid was not clear but dark yellow/green, and she is now experiencing contractions every 2 minutes.
Your bedside doppler reveals reassuring fetal heart tones at a rate of 152. On your initial exam, the cervix is fully dilated and effaced…and you see the baby’s head. As your obstetric colleagues are several minutes away, you check your pulse, dust off the radiant warmer, and prepare your resources for an ED delivery.
The mother delivers within the next few minutes with your OB colleagues still en-route. While she has minimal complications, the newborn is now not doing as well.
Neonatal Exam
Constitutional: Full term boy, covered in meconium
Skin: Appears blue gray, cool to touch
Respirations: For the most part apneic and at times grunting
CV: Heart rate 100
Tone: Flaccid
You take over the resuscitation of the neonate immediately as he is delivered given the above exam. Attempts at stimulation, drying, oral suctioning, and warming still yields a 1-minute APGAR of 3.
NRP/PALS
As per the algorithm below, with continued apnea and SpO2 in the 50’s you begin positive pressure ventilation and continued stimulation [1]. At 15 minutes, despite your attempts with continued PPV, suctioning, and stimulations, the SpO2 continues to be in the 60’s and the heart rate begins trending towards from 100 to the 60’s as well. You decide to intubate the patient, and the tube is passed successfully. Tube placement is confirmed and the x-ray shows fluffy infiltrates bilaterally in both lung fields but no evidence of barotrauma. Attempts at bagging yields minimal resistance, however the highest SpO2 the patient reaches is in the low 80’s at 40 minutes, with improved heart rate to the 120’s. At this point, your pediatric colleagues have found their way to the ED and are ready to take the neonate to the NICU. Two hours later into your shift, you read that the neonate was placed on veno-venous extracorporeal membrane oxygenation (ECMO) and is doing better. After your shift is over, you find your way over to the NICU and you see a pink sedated baby under a radiant warmer and connected to a large ECMO circuit.
Diagnosis: meconium aspiration syndrome complicated by pulmonary vascular hemorrhage and ARDS.
Had this neonate been in your ED, and your resuscitative measures have been exhausted, at what point do you consider ECMO in the neonatal or pediatric patient? I will review briefly the emergence of ECMO in the neonatal and pediatric literature, as well as its indications and the limited data on its utility in the emergency department setting.
From theory to practice
The first cardiac bypass circuit was created by John Gibbon in 1936 in the operating suite. ECMO is defined as a mechanical circuit outside the body where blood oxygenation and carbon dioxide removal can occur for patients with reversible cardiac or respiratory failure [2]. Through the 1950-1970s, many researchers studied various materials and techniques to further improve membrane oxygenation and attempted to increase the length of time a patient could remain on bypass. However, it was not until 1976 in which Bartlett et al. reported on the first neonate suffering from meconium aspiration syndrome to be successfully treated ECMO [3]. Since then, ECMO has grown and been used in various forms as treatment of respiratory failure in the neonatal and pediatric ICU settings. ECMO is to be considered for any reversible pathologic process that is impeding adequate tissue oxygenation and ventilation.
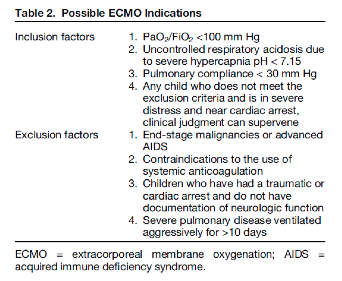
Indications for ECMO in children
Neonates:
Primary pulmonary hypertension of the newborn
Meconium aspiration syndrome
Persistent fetal circulation
Congenital diaphragmatic hernia
Pediatrics:
Respiratory failure
Bronchiolitis
RSV
Pneumonia
ARDS
Aspiration
Status asthmaticus
Sepsis
Cardiac arrest
with reversible conditions or amenable to heart transplantation
with favorable neurologic outcomes, some studies show better outcomes with CPR initiated in up to 95 minutes [5], key being excellent CPR with ETCO2 > 10 and early recognition and initiation of eCPR (extracorporeal cardiopulmonary resuscitation)
Hypothermia (cold water drowning)
In general, pediatric ECMO has been found useful in the above settings, however Gehrmann et al. reviewed the indications across various institutions when patients have been refractory to medical therapies. These indications are shown in the table below.
Types
ECMO comes in two types: venoarterial (VA) and venovenous (VV). While the early VA ECMO circuits utilized the roller pump to provide non-pulsatile flow of oxygenated blood, its limitations for infants included the necessity to cannulate the artery via either an open sternotomy or open cannulation of the carotids with the risk of losing a unilateral carotid artery due to ligation [4]. VV ECMO is the newer of the two modalities and rather than accessing a major vein and a major artery, the circuit requires access to either 2 major veins or a single major vein using a double-lumen catheter. However, each modality has its associated risks and benefits as described in the chart below [2].
Outcome Data
Neonates:
Neonates generally have better survival and outcomes on ECMO than older children and adults. One single center retrospective study in the journal of pediatric critical care published that of the 264 neonates placed on ECMO, 211 (80%) survived to discharge and 10-year survival was 71% [5]. The study had 2 cohorts and followed both neonatal and pediatric patients, collecting both demographic and survival data from patients placed on ECMO from 1987 to December 2013. Of those neonates that were alive at 90 days, at a 10-year follow up (ie.10-year conditional survival) 93% were alive. Of the common indications for neonatal ECMO, the 90-day survival and 10-year conditional survival are as follows respectively:
meconium aspiration syndrome (99% and 100%)
congenital heart anomalies (58% and 86%)
congenital diaphragmatic hernia (68% and 74%)
neonatal infection (72% and 100%)
Furthermore, patients who underwent VV ECMO had better 90-day survival and 10-year conditional survival rates than VA ECMO, 92% vs. 69%, and 98% vs. 87% respectively.
Pediatrics:
The same study followed 136 pediatric patients as well showing that the 90-day survival rate was 66% (90 patients) and that the 10-year conditional survival rate was 89%. The study divides the 90-day survival rates for ECMO by splitting it between pneumonia (bacterial, viral, and aspiration, 66, 76, and 83% respectively), other causes of sepsis (33%), trauma (100%), and other respiratory etiology (63%). Again, patients who underwent VV ECMO had better 90-day survival rates than VA ECMO, 78% vs. 50% respectively.
Other retrospective studies report similar survival rates to discharge when ECMO is used for severe respiratory failure, with rates from 39% to 83% depending on the pulmonary diagnosis, with status asthmaticus having survival rates of 83-100% on ECMO [2,6].
Data in ED
While data on emergency department utilization of pediatric ECMO is limited, one study mentions the outcomes from its two cases [7]. The first case was a 3-year-old previously healthy girl who presented with nausea and developed cardiogenic shock in the department from viral myocarditis. This patient was placed on ECMO in the ED and transferred to the cardiac intensive care unit. She was taken off the circuit after 4 days with improved contractility, and eventually discharged home 10 days later neurologically intact. The second case was similar in etiology: a 17-year-old male who presented in respiratory distress and suffered a PEA arrest. This patient achieved ROSC, was placed on ECMO in the ED and was eventually discharged home 31 days later with a favorable neurological outcome.
Conclusions
While some centers continue to grow their extracorporeal membrane oxygenation programs to expand their resuscitative efforts, still few places have the equipment to start the circuit or even at times an on-call staff to prime the machine. In general, few studies report the usage of ECMO in the emergency departments as a means for cardiopulmonary resuscitation of a reversible etiology, and only case reports exist in the pediatric literature. However, even if the ability to start neonatal/pediatric ECMO may not be ever be present in one’s emergency department, prompt recognition and early disposition to tertiary care centers that offer these services could potentially be lifesaving in many appropriate cases.
Expert Commentary
While ECMO (extracorporeal membrane oxygenation) may seem a foreign concept for ED management, it is important to recognize that as recently as the 2009 H1N1 epidemic, utilizing this intervention as a rescue therapy to treat acute severe hypoxic respiratory failure associated with influenza resulted in cases of ECMO initiation in emergency rooms across the world. Your review introduces meconium aspiration – a frequent clinical indication – as a platform to review this technology. Critical to the understanding of ECMO is first to be able to determine the appropriate candidates for this support, then to pick the correct mode (veno-arterial versus veno-veno) and to learn your own institution’s interpretations of inclusion and exclusion criteria. For the purposes of this commentary, we will break down the review into five sections sections: types of ECMO, who goes on ECMO, how to measure and trend hypoxemia, what can you do in the ED to maximize success on ECMO and new advances in ECMO.
ECMO modes:
ECMO is temporary cardiopulmonary bypass used to support a patient with either heart or lung failure. Originally developed from modifications of cardiopulmonary bypass and subsequently adapted for prolonged support, an ECMO circuit has both an oxygenator and a pump and a rate by which the blood passes across the oxygenator filter and has carbon dioxide removed. Children with heart or lung failure who do not respond to conventional medical therapies may be candidates for ECMO support. The ECMO circuit drains blood from the body, exchanges oxygen and carbon dioxide on the red blood cell, warms the blood and pumps it back into the body. Placing a child on ECMO allows time for the heart and/or lungs to improve or to serve as a bridge to other long-term extracorporeal support or transplant.
In this very simple model, the oxygenator functions as the lungs, the pump takes over the work of a beating heart and the flow of the blood across the oxygenator mimics a respiratory rate and removes the carbon dioxide. If a patient’s heart is still adequately functioning as a pump and the indications for ECMO are due to oxygenation and/or ventilation defects, then utilizing ECMO as an isolated pulmonary bypass is preferred and veno-venous cannulation is warranted. Blood is therefore removed from a vein, red blood cells are oxygenated and carbon dioxide removed, and then blood is returned to a vein for the heart to pump. This approach allows for an adequate mixed venous saturation and tissue oxygenation. More importantly it allows for the heart to perfuse the coronary arteries with antegrade flow, does not require that an artery be sacrificed for access, permits a pulsatile waveform to be sensed by organs and can often be performed with a single dual-lumen catheter. Most frequent sites are internal jugular vein or femoral vein. Cannulation can be accomplished by either using 2 separate cannulae or by utilizing a dual lumen internal jugular cannula that spans from superior vena cava (SVC) to inferior vena cava (IVC) in order to remove blood via end and side ports in the cavae and return blood via a side port of the second lumen that is positioned in the right atrium and directed towards the tricuspid valve.
If the patient has had any indications of cardiac injury including but not limited to cardiac arrest, arrhythmias, severe pulmonary hypertension, or evidence of significant cardiac dysfunction, then venoarterial cannulation is indicated as the patient’s heart can no longer be relied upon to pump blood.
ECMO Candidates:
The review elegantly lists criteria for candidacy but there are few others to be considered including mediastinal masses with airway compression and complex airway disease. Usually ECMO is on stand-by for these cases in the operating room.
Measurement of hypoxemia:
While many are using P/F ratio (as Steve commented on in his review), the gold standard remains calculating and trending oxygenation index as a surrogate marker for severity of hypoxemia. Patients with an OI > 25 x 6 hours are demonstrating refractory severe lung failure and should be considered for ECMO.
Pre-ECMO Studies:
If you have a patient scenario develop in the Emergency Department with a high likelihood of progression to ECMO, there are handful of tests/treatments to be ordered prior to initiating ECMO that will make the decision to proceed with ECMO easier for the inpatient team:
1. Is there any evidence of a CNS abnormality or bleed? Order a head ultrasound on the infant or a head CT on the child if able – you want to rule out any CNS bleed that would be worsened by anti-coagulation.
2. Does the child have an underlying congenital heart lesion? Order an ECG and ECHO on every patient – you want to rule out any congenital cardiac lesions and to quantify pulmonary hypertension.
3. Is there any evidence of pulmonary hypertension – regardless of whether this is acquired or idiopathic? If “yes” – then start iNO – you can presume that all mechanisms of respiratory failure are coupled with worsening pulmonary hypertension in the child.
4. Order a type and screen and a CBC. While not ideal, a baby with a hemoglobin of 14 g/dL can be placed on a saline-primed ECMO circuit emergently with the recognition that this will dilute and drop their Hg and interfere with oxygen carrying capacity. For smaller children where the total volume of the ECMO circuit can exceed their own intravascular volume, best to be able to prime the ECMO circuit with blood before placing the patient on the ECMO pump.
New Advances in ECMO:
The ECMO world is pushing the envelope and recognizing that preventing deconditioning and minimizing de-recruitment of both lung tissue and muscle mass is key to recovery or to eventual organ transplantation. Centers across the US and Europe now have their patients walking on ECMO. Who knows, one may walk into an ER near you some day!!
Kiona Allen, MD
Assistant Professor of Pediatrics, Cardiology
Leah Harris, MD
Professor of Pediatrics, Pediatric Clinical Care
How To Cite This Post
[Peer-Reviewed, Web Publication] Chukwulebe S, Lang S. (2019, April 8). Pediatric ECMO: Beyond the basics of pediatric resuscitation [NUEM Blog. Expert Commentary by Harris L & Allen K]. Retrieved from http://www.nuemblog.com/blog/pediatric-ecmo
Other Posts You May Enjoy
Resources
Pediatrics AA, Association AH. Textbook of neonatal resuscitation. 2006.
Gehrmann LP, Hafner JW, Montgomery DL, Buckley KW, Fortuna RS. Pediatric Extracorporeal Membrane Oxygenation: An Introduction for Emergency Medicine Physicians. J Emerg Med. 2015;49(4):552-60.
Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs 1976;22:80—93.
Mok YH, Lee JH, Cheifetz IM. Neonatal Extracorporeal Membrane Oxygenation: Update on Management Strategies and Long-Term Outcomes. Adv Neonatal Care. 2016;16(1):26-36.
Alsoufi B, Osman A, Nazer R, et al. Survival outcomes after rescue extracorporeal cardiopulmonary resuscitation in pediatric patients with refractory cardiac arrest. J Thorac Cardiovasc Surg 2007; 134:952–9.
Swaniker F, Kolla S, Moler F, et al. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg. 2000;35(2):197-202.
Nevet A, Polak T, Dagan O, Waisman Y. Extracorporeal Membrane Oxygenation as a Resuscitation Measure in the Pediatric Emergency Department. Isr Med Assoc J. 2015;17(10):639-41.